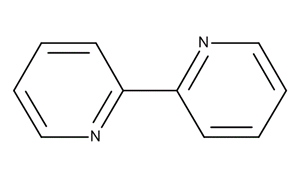
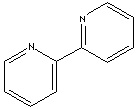
2,2'-Bipyridine
- 2,2 '- bipyridinyl
- α -bipyridyl
- α, α' -dipyridyl
- Bipy
White crystals
Fixed
70-72 ° C
272 ° C
Poorly in water ( 5.5 g · l-1 at 22 ° C)
Risk
100 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
2,2 '-bipyridine is a chemical compound selected from the group consisting of bipyridines, with molecular formula C10H8N2. It consists of two pyridine rings that are respectively in α - position linked to one another.
Representation
2,2 '- pyridine can be synthesized from pyridine at 330 ° C and iron ( III) chloride, or at room temperature with Raney nickel. Another simple synthesis is the Ullmann - coupling of 2- bromopyridine, is used for copper dust.
Properties
2,2 '-bipyridine is a white solid which melts at 70-72 ° C and boils at 272 ° C at room temperature. The compound can be used as a bidentate chelating ligand, and thereby coordinated on the nitrogen atoms. As a ligand, the short notation is used bipy often. With many transition metals form stable complexes are formed. In an octahedral complex geometry produces two enantiomeric complex species.
Use
The complex of 2,2 '- bipyridine with ferrous iron ( [Fe (bipy ) 3] 2 ) is deep red in solution. This complex can be used for the quantitative colorimetric determination of iron. Ferric iron can predict, for example, sodium bisulfite can be reduced.