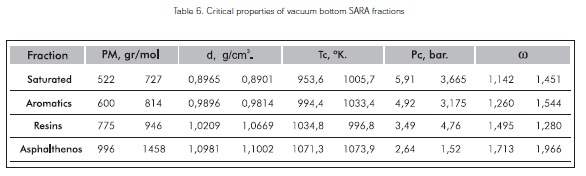
Acentric factor
The acentric factor by Pitzer, also called Azentrizitätsfaktor, is a dimensionless material size. It is used in thermodynamics as a measure of the deviation of a molecule from the ideal spherical shape, and is mainly used in the thermal condition equations for material gases, such as Soave - Redlich - Kwong equation of state or in the state equation by Peng -Robinson.
Definition
The acentric factor is defined as:
- Tr = T / Tc - reduced temperature
- PS - saturated vapor pressure at Tr = 0.7
In order to
- If it is,
- If it is,
- When.
Are for a substance, the critical temperature, the critical pressure and the boiling point at atmospheric pressure is known, then the acentric factor can be approximately calculated by:
At the temperatures are absolute temperatures.
For substances whose molecules differ only slightly from the spherical shape (eg methane ), applies.
The Azentrizitätsfaktor was originally used by Pitzer as an expression in the equation for the compressibility. By adapting to the experimentally determined vapor pressures of hydrocarbons, the equation is quite accurate at this.