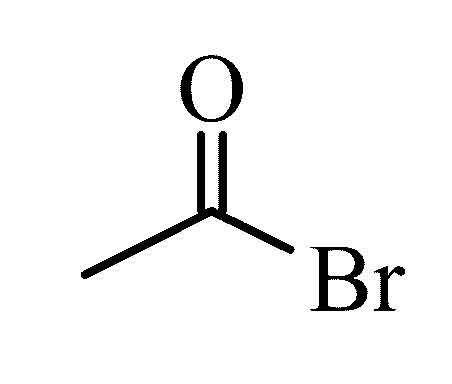
Acetyl bromide
- Essigsäurebromid
- Acetoxybromid
Volatile, yellowish liquid with a pungent odor
Liquid
1.65 g · cm -3
-96 ° C
76.7 ° C
133 mbar ( 20 ° C)
Decomposes in water with vigorous reaction
1.4537 (at 16 ° C, 589 nm)
Risk
-223.5 KJ / mol
Template: Infobox chemical / molecular formula search available
Acetyl bromide is a chemical compound from the group of carboxylic acid halides and organic bromine compounds.
Production and representation
Acetyl bromide can be prepared by reaction of phosphorus, and acetic acid:
Properties
Acetyl bromide is a volatile, colorless to yellowish, fuming liquid with a pungent odor in the air. When heated or in contact with water or lower alcohols it decomposes with violent reaction, hydrogen bromide is formed.
Use
Acetyl bromide is mainly used as the acetylating agent in organic synthesis.
Safety
The vapors of acetyl bromide can combine with air to form an explosive mixture (flash point 75 ° C).