Adhesion
Adhesion (Latin adhaerere, stick '), also known as adhesion or attachment force is the physical state of an interface layer that forms come into contact between two condensed phases - that is, between solids and liquids with negligible vapor pressure. The main feature of this state is caused by molecular interaction at the interface layer of mechanical cohesion of the phases involved. The mechanical integrity of these effecting forces are not all fully understood, which is why there are different Adhäsionstheorien.
- 2.1 Adhesion in transport
- 2.2 Adhesion in adhesives
- 2.3 adhesion for films
- 2.4 Adhesion in organic tissues
Adhäsionstheorien
A distinction is generally BASED between mechanical adhesion due to physico - mechanical forces and specific adhesion to chemical, physical and thermodynamic basis of forces for which there is in each case different Adhäsionstheorien. These theories were developed individually, but according to current knowledge and specific adhesion mechanical form a unit.
Mechanical adhesion
The theory of the mechanical adhesion refers to Verklammerungen an adhesive in the microscopic pores and cavities of a solid body. Previously, this was the only explanation for adhesion; the question of the cohesion between a solid with a smooth surface and adhesive can therefore not answer.
Specific adhesion
Theories of specific adhesion have been developed, because the theory of mechanical adhesion was not sufficient to explain the cohesion of solids with smooth surfaces. The various theories of specific adhesion and the mechanical theory of adhesion are not mutually exclusive, but complementary.
The polarization theory ( De Bruyne 1935) According adhesion is based on the dipole character of the molecules. This explanation, however, is limited to polar substances.
Electrostatic theory ( Derjagin 1950) is an electric double layer ( a plurality of molecular or atomic layers thick space charge region caused by charge displacements ) as the cause of the force of adhesion to. However, it must be suitable carriers such as electrons or ions, can be present.
Diffusion theory ( Voyutzkij 1960) sets the Brownian motion (ie, the temperature caused by the self-movement of the molecules ) basis, which results in that the particles of both materials diffuse into each other parties. However, the materials must have a chemical affinity to each other, which is usually the case only with plastics. For metals as the metal binding prevents diffusion.
Basis of adsorption and wetting theory ( Zismann, Fowkes, Good and Wu 1963), the surface and interfacial surface theory. This thermodynamic consideration of the adhesion after wet solid surfaces such liquids particularly well, which are located at the interface with the solid phase in a slightly less favorable energetic state than in the interior. If the energy state at the phase boundary cheaper than in the interior, it comes to the complete wetting ( also called spreading ), stick all the particles of the liquid on the solid surface at the. No wetting - the other extreme - occurs when the energy state is favorable for the particles inside the liquid, that it forms a ball, reducing the area of contact with the solid material to a minimum. Taking into account the structure of the interface layer (roughness and impurity particles), temperature and other factors on the thermodynamic consideration, it is thus draw conclusions on the adhesion.
Occurrence
Adhesion in transport
Among adhesion is understood in the road the road grip ( grip eng. ) - Rubber (tires) to ground ( road ) - and in rail transport rail adhesion - iron ( track bikes ) to iron ( rail). From an adhesion line is when a train without aids can cope with steep gradients (eg gear or rope) and only sufficient adhesion of the wheels for locomotion. Adhesion is also involved in launching aircraft boats and withstands the hull in the water. The designers solved this problem by means of a tear-off edge at half the length of the keel (also called stage), so that the hull of the machine can detach from the surface.
Adhesion with adhesives
Adhesion comprises the adhesive forces at the contact surfaces of two different or identical materials by molecular forces. The materials can be in solid or liquid state. In the field of adhesives is meant by adhesion, the adhesion of adhesive layers to the adherend surfaces. The processes of adhesion are not yet fully understood. They make particularly difficult because the dependencies between the adhesive systems and the various adherend surfaces are very complex.
Adhesion for films
Cling adhere without adhesive on smooth / glossy surfaces by means of the attraction of the molecules between the two materials. The prerequisite is that the molecules are as close as possible to achieve adhesion. Therefore, this only works on smooth surfaces, such as a protective film on the display or tinting films on glass from motor vehicles.
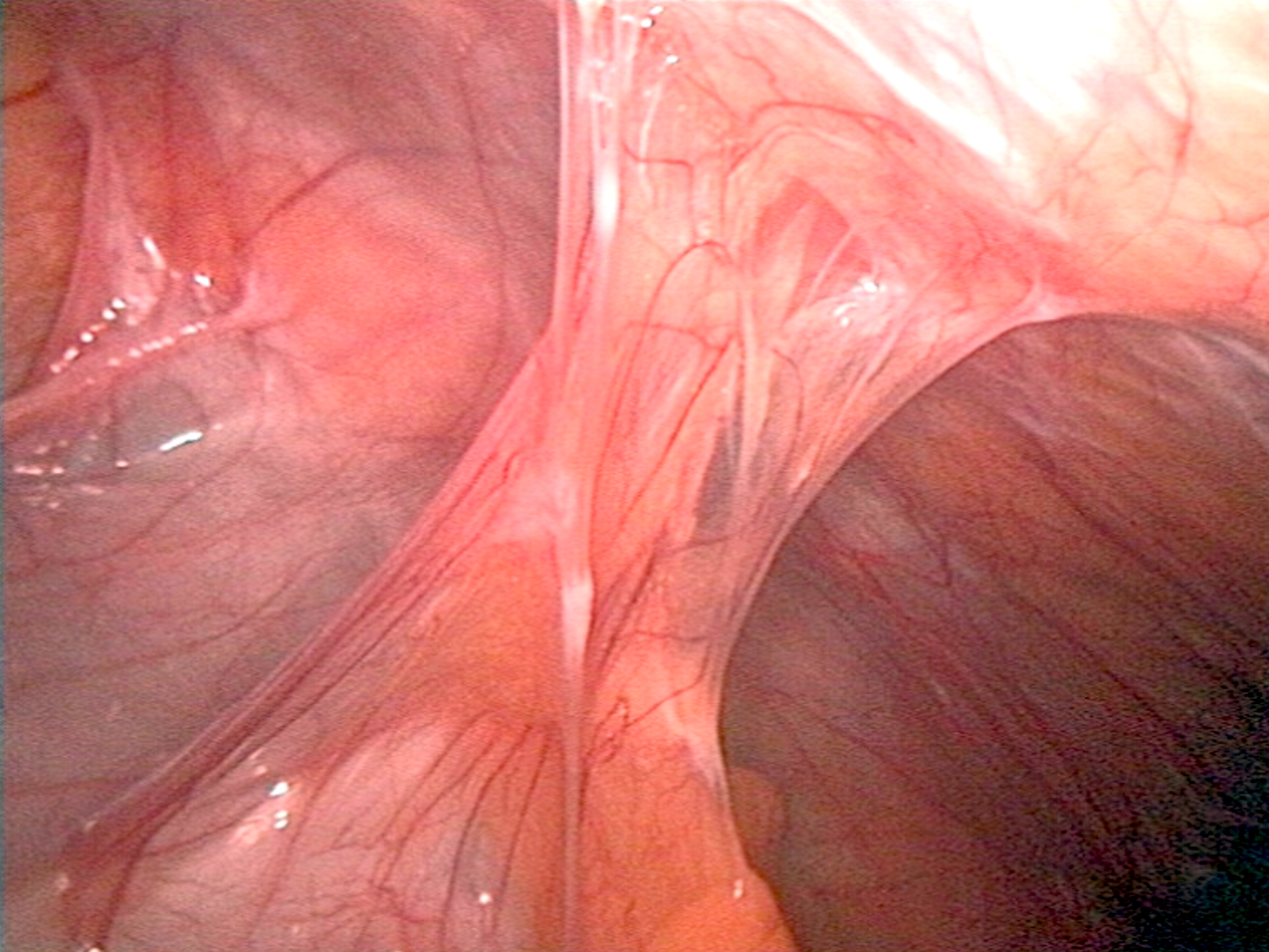
Adhesion in organic tissues
- Surgically: adhesions or adhesions of organs (see Verwachsungsbauch )
- Haematological: adhesion of blood cells (eg, platelets, leukocytes) to both internal vessel walls ( eg in the context of an inflammatory response ), as well as vessel inner wall foreign ( endothelfremden ) surfaces or tissues
- Bacteriologically: Adhesion of bacteria to mucosal surfaces at the beginning of infection
- Cell adhesion: Adhesion of tissue cells to proteins of the extracellular matrix on focal adhesion sites and hemidesmosomes
- Dental: Chemical adhesion of restorative materials to dentin ( dentinadhesiv )
- Adhesion of a complete denture on the mucosa.