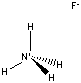
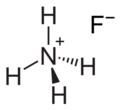Ammonium fluoride
- Ammonium fluoride
- Ammonium fluoride
White, deliquescent crystals
Fixed
1.01 g · cm -3
Decomposition above 100 ° C.
Well in water (820 g · l-1 at 20 ° C)
Risk
1 mg · m -3 ( inhalable aerosol fraction)
Template: Infobox chemical / molecular formula search is not possible
Ammonium fluoride ammonium salt of hydrogen fluoride.
Properties
Ammonium fluoride is colorless, slightly deliquescent, easily soluble in water and is toxic crystals.
Reactions
When heated, it decomposes into ammonia and hydrogen fluoride:
Synthesis
The preparation of ammonium fluoride is carried out by neutralization of aqueous ammonia solution with hydrofluoric acid, followed by crystallization from water.
Another synthetic route is the heating of ammonium chloride, sodium fluoride, wherein the product removed by sublimation:
Precautions
Ammonium fluoride is toxic. Avoid contact with skin and eyes. Contact with strong acids, hydrogen fluoride is released. This is extremely toxic and highly corrosive.
Disposal
Dissolved fluoride as calcium fluoride precipitate.