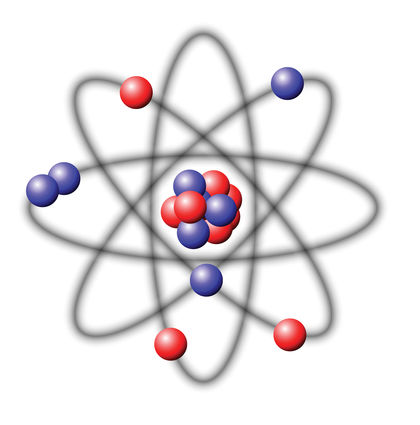
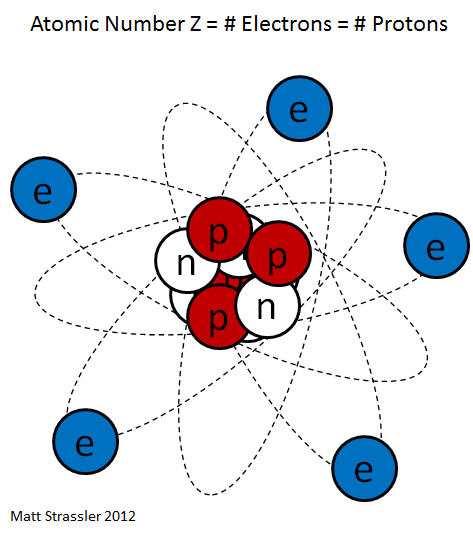
Atomic nucleus
The nucleus is, in comparison to the atomic shell, tiny core of the atom.
Knowledge of the properties of atomic nuclei, for example, for understanding the radioactivity, nuclear fission ( nuclear power plant, nuclear reactor, nuclear weapon) and nuclear fusion (nuclear fusion reactor, hydrogen bomb ) is necessary, but also magnetic resonance imaging ( MRI) in medicine.
From the Latin word nucleus ( walnut or pine nut ), the term derives " nuclear" from. He called things or effects that are associated with properties or with reactions of atomic nuclei, such as nuclear medicine. Different types of atoms are labeled according to the structure of their nuclei as nuclides.
Structure of the atomic nucleus
Size, density, components, names
The nucleus is located, spoke clearly, in the center of the atom; its diameter is about 1/10.000 to 1/100.000 the diameter of the electron shell, but focuses in more than 99.9 % of the mass of the entire atom.
The density of the core ( the ratio of core mass to core volume ) is approximately equal for all nuclei and is approximately 2.1017 kg / m³, water that is slightly superior to the 2.1014 times.
The core consists of protons and neutrons, which are collectively called nucleons also. The number of protons is the atomic number, the total number of nucleons mass number of the nucleus called ( for details about the mass of the nucleus see core mass or mass defect). The mass numbers of the naturally occurring atoms on earth vary from 1 (hydrogen) to 238 (uranium ). The macroscopic density of condensed matter, however, varies much less because the atomic radius is determined by the atomic shell, the stronger the chemical nature of the atoms varies as the mass number. Nevertheless, includes so-called heavy atomic nuclei also colloquially / technical heavy elements. For example, lithium ( mass numbers 6 and 7 ) has a density of 0.53 g / cm ³, gold ( mass number 197 ), however, of 19.3 g / cm ³.
Protons are positively electrically charged, uncharged neutrons. Therefore, the atomic nucleus is positively charged and can bind through the Coulomb negatively charged electrons in itself. Since the electric charge of the electron from its sign is equal to the charge of the proton, an electrically neutral atom outwardly as many electrons in the atomic shell must have as protons in the nucleus. Since the atomic shell largely determines the chemical properties, the atomic number also specifies to which element the atom belongs, it is the chemical atomic number. Neutrons have about the same mass as protons. Number has only little influence on the chemical properties of the atom, but is critical to the stability or instability of ( radioactivity) of the core. Be by chemical or physical effects, electrons added or removed, the atom is electrically charged from the outside and is called ion. In the nucleus, this changes nothing. Apart from the radioactivity, the number of protons or neutrons in the core may vary only by a nuclear reaction.
A fixed by atomic number and atomic mass number or atomic nuclear species is called nuclide. When isomers are atomic nuclei in long-lived excitation levels of the core ( see below) referred to; they count as separate nuclides. If we distinguish nuclei ( or whole atoms) of the same element, ie with the same number of protons, according to their number of neutrons, one speaks of the isotopes of the element. Are referred to nuclides with the chemical element symbol and the mass number, such as the common carbon isotope 12 C or the most frequent iron isotope 56Fe ( in isomers or with an additive such as "m" for " meta-stable "). Common, but is also the redundant notation with mass number and atomic number.
There are a total of about 3200 long-lived nuclides known, spread over about 2700 isotopes and 118 known elements from hydrogen to Ununoctium (as of 2003). Among them, there are about 250 stable isotopes. The stability of a nuclide is dependent on the number of protons and neutrons. If the number of protons equal to 43 or 61, or greater than 82, or is the ratio of the two numbers unfavorable, the core is unstable, ie radioactive, and is transformed into a more stable core.
Nuclear and Coulomb
The positively charged protons in the nucleus repel each other due to the Coulomb force from. Since the core but still does not fly apart, another force must exist, with an eye towards the nucleons each other and that is stronger than the Coulomb force in the core. This force is called the nuclear force (not to be confused with the colloquial term " nuclear power " for nuclear energy); it is a residual interaction of the strong interaction. Nuclear power is still only approximately described. Your education is among other things the subject of nuclear physics. However Secured is their very short range of the nuclear force, which is of the order of the nucleon diameter ( about 1 fm = 10-15 m). It ensures that there is no arbitrarily large cores, for a proton on the "surface " of a large core attraction only senses of its nearest neighbor nucleons, Coulomb repulsion, however, from all other protons of the core. Are a sufficient number of other protons present, therefore eventually outweighs the repulsion. The number of protons lead 82 is the last element with stable isotopes, all others are radioactive (see decay chain ). The highest observed in natural resources nuclear charge is 92 (uranium ). Nuclei with more protons ( transuranic ) decay so quickly after their formation that they can only be observed after artificial production by a nuclear reaction.
Binding energy
The binding energy corresponds to the work that would have to be expended to break the core into its individual nucleons. Conversely, the binding energy would be released if it were possible to put together a nucleus of free protons and neutrons. Because of the equivalence of mass and energy, the formation of the core leads to a mass defect. That is, the atomic nucleus has less mass than if one adds the mass of its nucleons.
The binding energy per nucleon is different in different nuclei. On differences in the binding energy per nucleon of energy gain or loss based on nuclear reactions, ie in particular the possibility to gain energy on an industrial scale of nuclear reactions.
Energy levels
Nuclei have discrete energy levels like atoms. ( The result of this level is also above the binding energy of a nucleon still continues, as reflected for example in the resonances of the excitation function of nuclear reactions. ) An undisturbed core is typically located in its lowest energy level, the ground state. The higher levels ( excited states ) are not stable, but the core is sooner or later from there in a stable state, usually the ground state, the energy difference is emitted as photon ( gamma radiation). Particularly long-lived ( metastable ) excited states are called isomers.
Radioactivity
The term radioactivity refers to the property of unstable atomic nuclei spontaneously convert while releasing energy. Most of the elements exist only a few stable isotopes, or even only one, with atomic numbers 43 ( technetium ), 61 ( promethium ) and all above 82 ( Lead ) there is no stable isotopes.
In the unstable atomic nuclei essentially three types of decays can be distinguished:
- Decay with emission of nucleons (eg, alpha decay ),
- Conversion with emission of electrons or positrons ( beta decays ) and
- Transition between two states of one and the same nuclide with emission of gamma radiation.
Because of the above mentioned existence of short-range attractive and long-range repulsive forces in the nucleus, the binding energy per nucleon decreases toward high mass numbers. Therefore, occurs in some nuclides with mass numbers above about 140 alpha decay, above about 230 also Spontaneous fission. Both decay modes lead to nuclides with lower mass numbers.
In beta decay an electron or positron is emitted from the nucleus of a radionuclide. This is created by converting at the core of a neutron into a proton, an electron and an antineutrino and a proton into a neutron, a neutrino and a positron. The sum of the electric charges and the number of nucleons is preserved, but the chemical atomic number changes by itself.
The emission of gamma radiation requires that the nucleus is in an excited state (see section energy levels ) and therefore mainly occurs immediately after an alpha or beta decay on, if it does not lead directly to the ground state of the daughter nucleus. Therefore, the gamma emission analogously to other processes, the radioactivity is sometimes referred to as gamma ' decay '.
Core models
In nuclear physics, no single model for the comprehensive description of all operations there in the nucleus. Compared to atomic physics with the successful quantum mechanical model of the atom missing in the core a special, massive force center, and the forces between nucleons are much more complicated than the purely electromagnetic interaction in the atom. Therefore, various nuclear models for different questions are used. The most important are:
- The droplet model (Carl Friedrich von Weizsäcker 1935, Niels Bohr 1936) describes the atomic nucleus to be spherical in round droplets of an electrically charged liquid and gives a formula for its total binding energy. With this almost classical model can be well explained which isotopes are stable and which can still transform by delivering their energy in a fixed bound (or two) is, for instance by α -decay, β -decay, nuclear fission. This will allow addressing also finds the number of different chemical elements on earth a justification.
- The shell model for nuclei ( Eugene Paul Wigner, Maria Goeppert- Mayer, J. Hans D. Jensen, 1949) leads the development of the atomic nuclei in analogy to the shell model of nuclear physics purely on quantum mechanical laws ( orbitals in a potential well, Pauli principle) back. The interaction between two nucleons is taken into account only in a further refinement. The shell model can explain the stability of the nuclei well, where it differs from the liquid-drop model, in particular the high stability of certain so-called magic numbers of protons and neutrons numbers. It also provides a detailed explanation of the energy levels, the nuclear spin magnetic moments mechanisms of nuclear reactions, in so far as they result from the movement of a single, or very few nucleons of the core. But excited states of a nucleus with the participation of many or even all nucleons are often formed.
- In the collective model ( Aage Niels Bohr, Ben Mottelson, 1953), the core is not an exact spherical shape, but is slightly flattened or stretched, as if it had been shown by the electric quadrupole moments of many nuclei in one direction. So excited states are possible in the form of collective vibration and collective rotation, in which so involve all nucleons. Result is a characteristic level diagram of the excited states in the form of the vibration band or rotational band.
- In the combined model (unified model, James Rainwater 1957) are connected shell model and the collective model.
In addition to these models, there are more that are used on more specific purposes:
- Fermi-gas model (also uniform model). Here the nucleons are accepted despite their strong interactions as freely and are only subject to the Pauli principle. This idea is used in the droplet model for binding energy to justify the asymmetry energy, which determines the ratio of the neutron to proton number.
- Alpha-particle model. Alpha particles are stable here subunits within the core of what makes a useful conceptual model, for example, for the cores C-12, O- 16, Ne -20.
- Potential well model. Here a certain potential is given and to calculate the energy eigenstates of a single nucleon in analogy to the atom. It is the basis of the shell model and the spatially limited Fermi-gas model. As forms of the potential, especially the simple square well potential, the oscillator potential, as well as significantly more realistic Woods - Saxon potential occur.
- Optical model. Here nuclear reactions are modeled by the fact that the fly- projectile is affected by the target nucleus as a light wave by an absorbing ( " cloudy " ) lens. The model is well suited for elastic scattering and for reactions in which the target nucleus is only taken from a particle or added to it.
Two opposite simplistic starting points show up on the models of the atomic nucleus:
- Model strong correlation: The nucleus is seen as a collection of tightly paired nucleons (eg droplet model, alpha-particle model);
- Models of independent particles: The nucleons move relatively freely in the nucleus ( Fermi-gas model, optical model, shell model potential well model).
Realistic models are distinguished by an appropriate combination of both approaches.
Between the individual models, the following relations can be established:
Each of these models is applicable only for a specific nuclear domain of phenomena. There is no consistent theory that includes all nuclear phenomena.
History
The key experiment that led to the discovery of the atomic nucleus, the doctoral student Ernest Marsden succeeded in the laboratory of Nobel laureate Ernest Rutherford on 20 December 1910. In control experiments for producing a sharply defined beam of α - particles, he had noticed that the particles by thin metal foils, although pass to 99.99 %, almost without distraction and, in isolated cases, but also by more than 90 ° are distracted. The strong deflection was contrary to the expected result: After Thomson 's atomic model then adopted ( " raisin cake model," English "plum pudding model" ) from the atomic electrons would have insisted that floated in a diffuse positively charged cloud. It was known that α - particles ionized atoms of the noble gas are helium and could deviate so far from their path by either the positively charged clouds still numerous collisions with the electrons. Purpose of the trials it was actually to investigate the properties of this cloud closer. Rutherford interpreted the unexpected result so that the atoms of the film largely consisted of empty space that could happen to the alpha particles freely, while small, electrically charged and very massive particles existed in the alpha particles in one of the rare particularly close collisions greatly from could throw their path. Short rough calculations showed Rutherford that these " cores " had to be at least 1000 times smaller than the atom, but contain virtually all of its mass.