Binodal
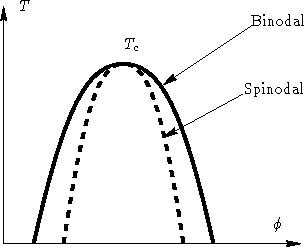
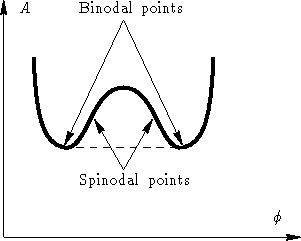
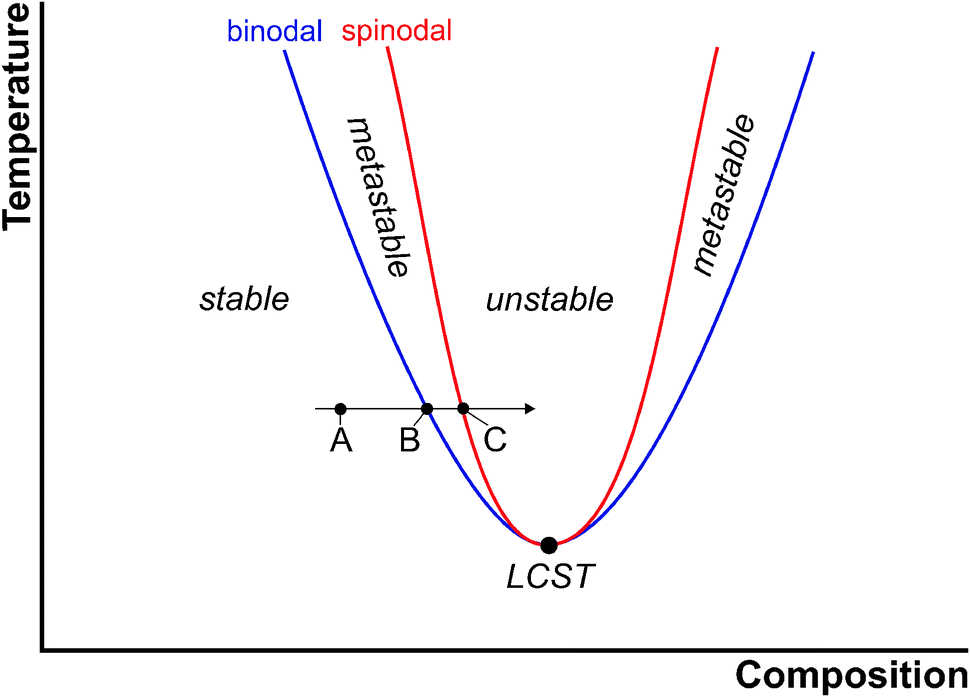
As a binodal ( coexistence curve also called ) is that of curve called in a phase diagram, from which a phase transition can take place and is thermodynamically favorable. It thus represents exactly the line of coexistence of both phases represents the binodal encloses the spinodal with which they may have common, so-called critical points ( see illustration).
Importantly, this concept is for example the case of binary mixtures. If you are in a suitable temperature range, by gradually increasing the concentration of a substance ( motion on a line parallel to the composition axis, ie on a tie line ), a point will be reached where the two substances not mix homogeneously, but to separate into two phases that coexist side by side ( miscibility gap ).
For conditions within the two phase spinodal states are the states of equilibrium ( minimum of Gibbs free energy ), as well as they are thermodynamically more stable ( the second derivative of the free energy, for example, on the concentration is positive). For the states between spinodal and binodal of the two-phase state is thermodynamically also preferred (minimum free energy ), but is the state without segregation only metastable ( stable against small displacements ), since the second derivative of the free energy is positive.
- Thermodynamics