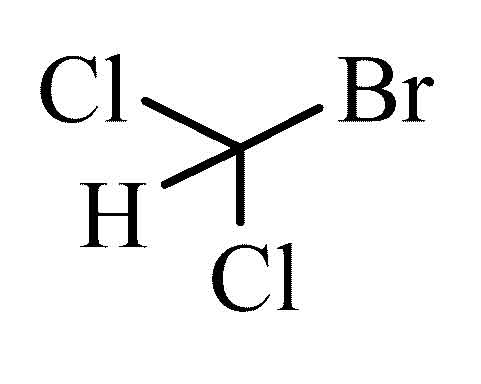
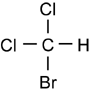
Bromodichloromethane
- Monobromdichlormethan
- BDCM
Colorless liquid
Liquid
1.98 g · cm -3
-57 ° C
90 ° C
67 hPa ( 20 ° C)
4.5 g · l-1 at 20 ° C.
1.4964 (20 ° C)
Risk
Template: Infobox chemical / molecular formula search available
Bromodichloromethane is a chemical compound selected from the group consisting of aliphatic, saturated halogenated hydrocarbons (specifically trihalomethanes ).
Occurrence
Bromodichloromethane occurs as a secondary product of the chlorination of water. Bromodichloromethane industrially is not used.
Properties and Uses
By heating in the presence of oxygen bromodichloromethane or reducing agents as the catalyst metals and slow decomposition takes place with the formation of acid gases (HCl, HBr). This reaction can be used for the production of long-lived halogen lamps.
Safety
Bromodichloromethane is classified as Carcinogen Category 2 and Category 3B Mutagenicity.