Bromoform
- Tribromomethane
- Methenyltribromid
Colorless chloroform similar smelling liquid
Liquid
2.8899 g · cm -3 ( 20 ° C)
9.2 ° C
149.5 ° C
- Poorly in water ( 3.2 g · l-1 at 30 ° C)
- Well in ethanol and diethyl ether
1.5948 (25 ° C)
Risk
1147 mg · kg -1 ( LD50, rat, oral)
-22.3 KJ / mol
Template: Infobox chemical / molecular formula search available
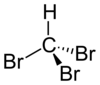
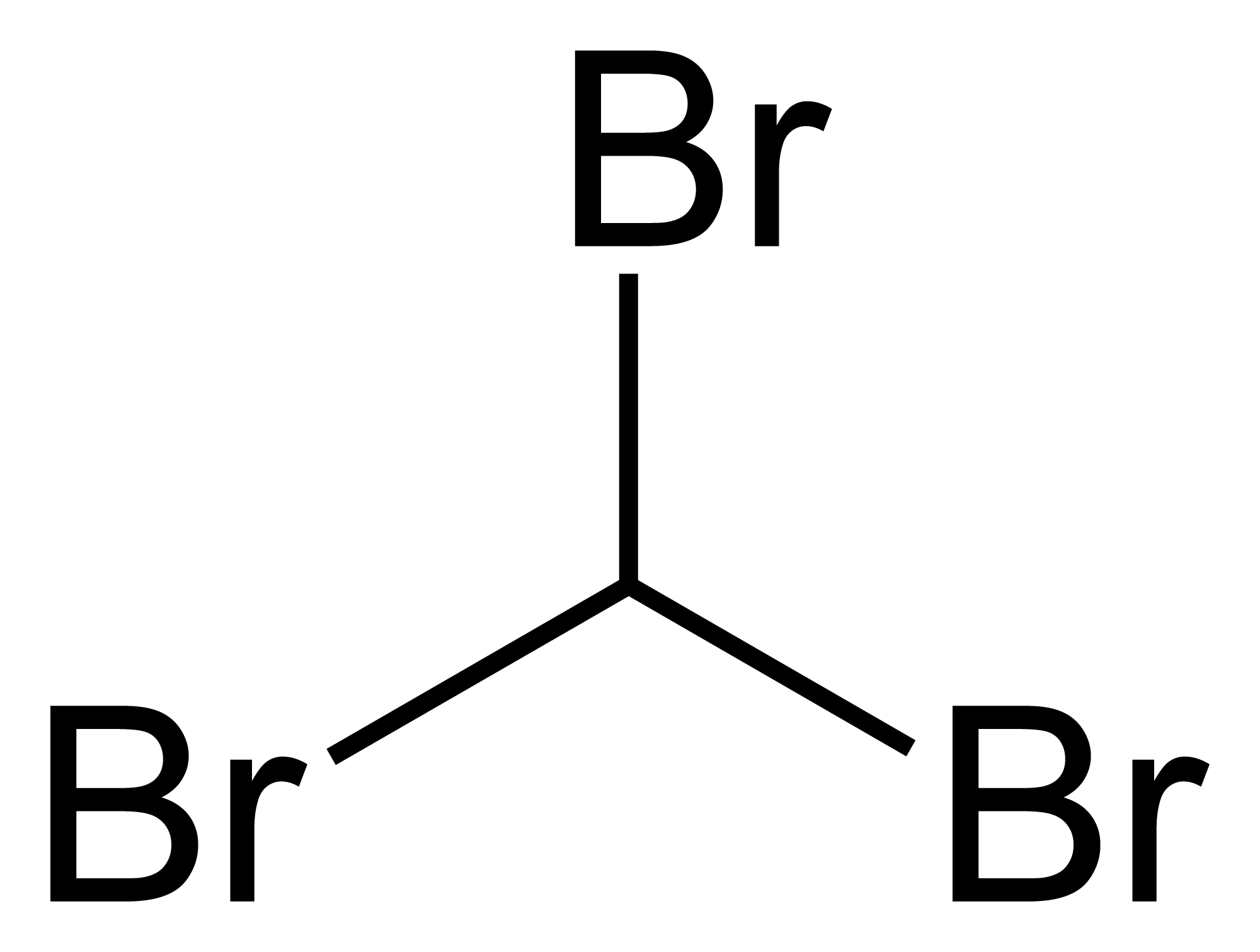
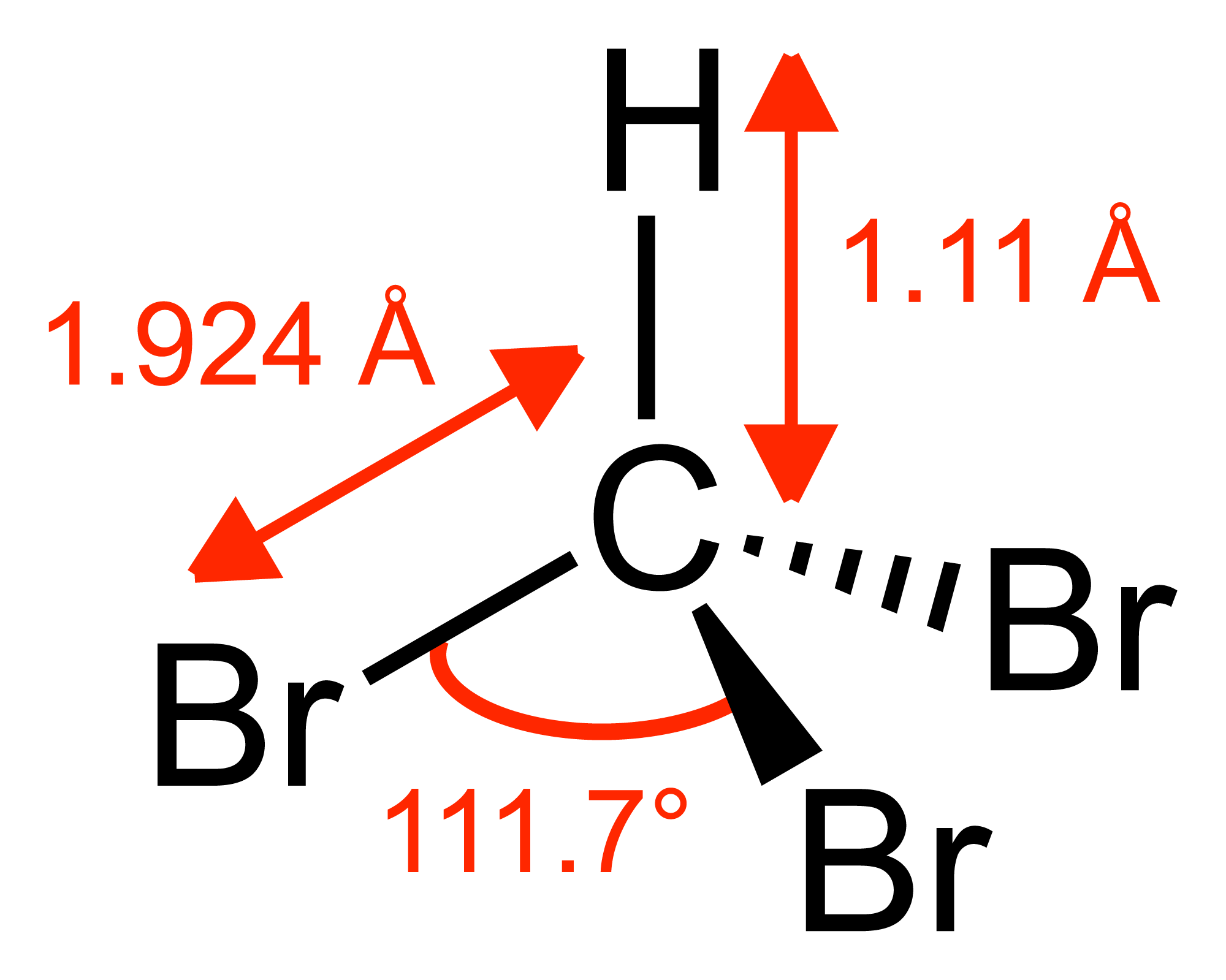
Bromoform ( CHBr3 ) is a chloroform -like halogenated hydrocarbon.
Occurrence and formation
Bromoform is produced from sea water due to its natural bromide. It is thus the largest source of organic bromine in the atmosphere.
It also arises in the treatment of water for cooling, bathing and drinking purposes and is included in all chlorinated and ozonated water. Coastal power plants use for cooling purposes, the chlorinated seawater, constitute the strongest produced by human source
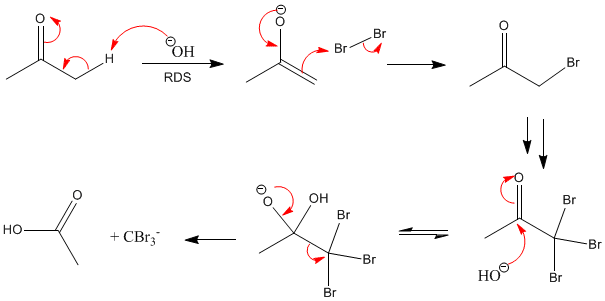
Bromoform may be prepared in the laboratory by the known haloform reaction, for example, from acetone (R = CH3) and hypobromite:
Properties
Bromoform reacts with many organic compounds, toxic bromine compounds may arise. Alkali metals, it reacts explosively. The vapor is heavier than air. When heated above the boiling takes place auto-decomposition.
Atmospheric behavior
From the short-lived in the atmosphere connection bromine radicals are released, which are responsible for photochemical processes in the troposphere and stratosphere. They play a role in the formation of the ozone hole among others.