Burgess reagent
Methyl -N-( triethylammoniumsulfonyl ) carbamate, inner salt
Colorless acicular crystals
Fixed
70-72 ° C
Attention
Template: Infobox chemical / molecular formula search available
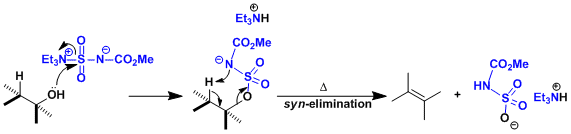
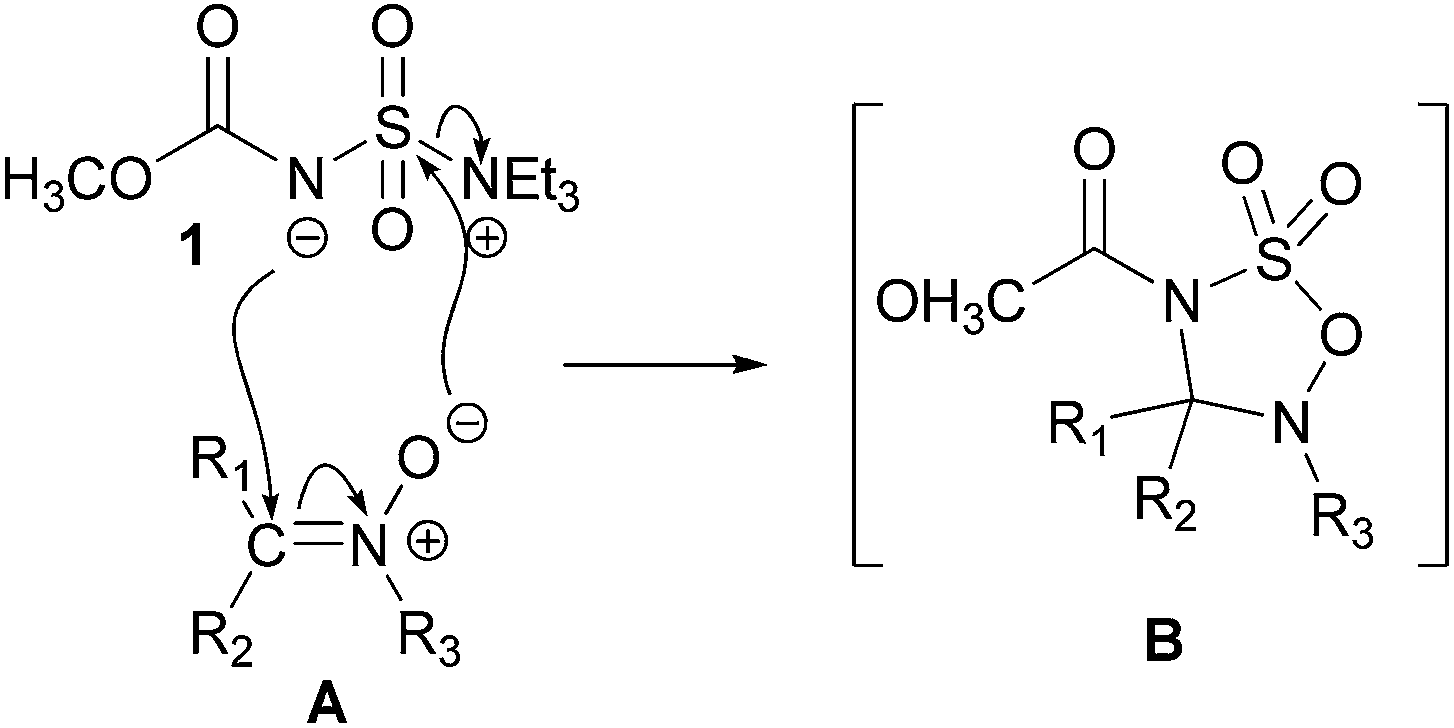
The Burgess reagent ( IUPAC name: ( methoxycarbonylsulfamoyl ) triethylammonium hydroxide ) is a very mild and syn - selective dehydrating reagent.
Production and representation
The methyl derivative described here, which is now commercially available under the name Burgess reagent, was first produced in 1970 by Crabbé and Léon. The synthesis was carried out, however, after a work rule which was published in 1968 by Burgess for the ethyl derivative. The reagent described here is made of chlorosulfonylisocyanate with methanol and triethylamine in benzene. For the ethyl derivative is carried out according to a similar process with ethanol instead of methanol.
Use
The Burgess reagent is suitable for synthesis of alkenes from alcohols. It also serves well for the construction of heterocycles.