Carbonyl
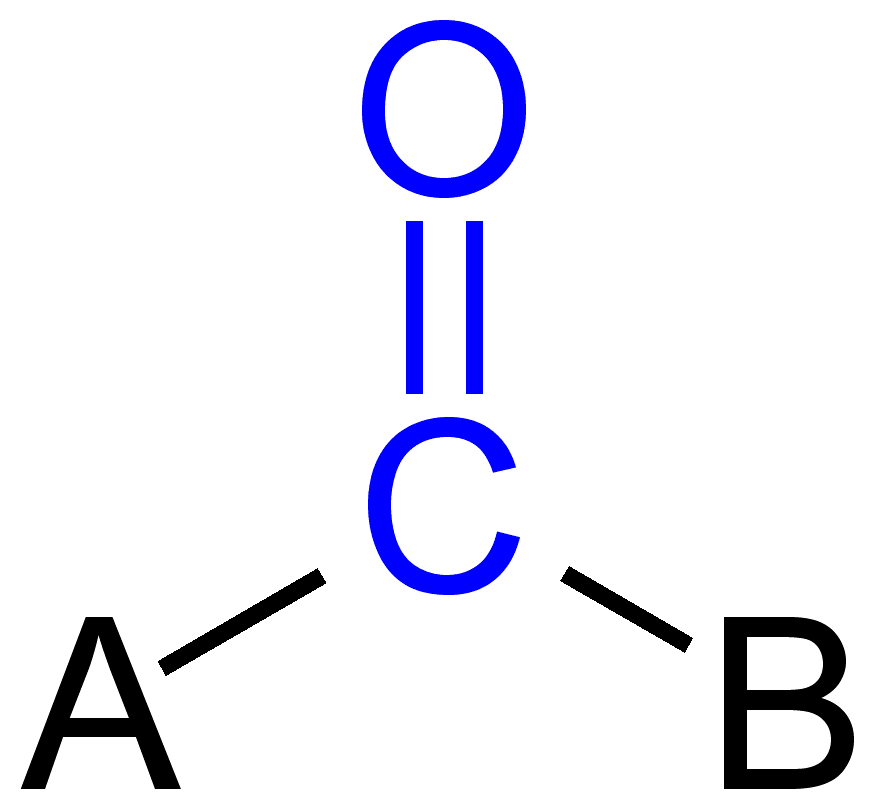
General structure of a carbonyl compound ( carbonyl group shown in blue ): Ketones: A, B = organic radical Aldehyde: A = H, B = organic radical or H Carboxylic acid: A = OH, B = organic radical or H Ester: A = O -R, B = organic radical or H Amide: A = NH 2, NHR, NR.sup.1R.sup.2, B = organic radical or H Ureas: A, B = NH 2, NHR, NR.sup.1R.sup.2 Urethane: A = OR, B = NH 2, NHR, NR.sup.1R.sup.2
The carbonyl group and CO group, is a component of many organic chemical compounds. It is characterized by a carbon atom ( the carbonyl carbon ), which carries a doubly attached oxygen atom (carbonyl oxygen ); similar sounding carboxy addition to the same carbon atom has a hydroxyl group, whereby a carboxylic acid is formed. If a molecule contains a carbonyl group, it is also designated as the carbonyl compound. Transmits only the carbonyl radicals, the group is also known as keto group. The monovalent organic radical which results formally from, for example, an organic acid by removal of the hydroxy group, is known as acyl group. An example of an acyl group as derived from the acetic acid is the acetyl group. The force of the electrophilic carbonyl carbon atom, and thus the reactivity of the compound to be substantially influenced by the properties of the substituent (A). Increase substituents with - I effect the reactivity, I and M substituents reducing.
The carbonyl group is present in, among other things:
The carbonyl group is similar to the sulfone group, there are many analogues, such as sulfonic acids or sulfonamides.
Carbonyl
In inorganic complex compounds (see, metal carbonyls ) denotes a carbonyl carbon monoxide ligand, which is coordinated by the C- atom to the central atom. Examples are the nickel tetracarbonyl, systematically tetracarbonylnickel ( 0) Ni ( CO) 4, further bridging the carbonyl can also occur in cluster compounds, as in Co2 ( CO ) 8 The carbonyl ligand is a strong aufspaltender ligand and binds synergistically to many metal ions, since it is a sigma -donor of two electrons and a pi - acceptor. He cleaves the ligand field on even stronger than the cyanide ligand CN.
Reactivity of the carbonyl
Carbonyl activity
Because of their polarity ( the carbonyl carbon atom has a positive, the carbonyl oxygen atom, however, a negative charge ) is the carbonyl group often responsible for the physical and chemical behavior of molecules containing them. Due to the electronegativity the LUMO ( lowest unoccupied molecular orbital ) is relatively low in energy and can be attacked by nucleophiles better. Moreover, this leads to carbonyl compounds usually have a higher melting and boiling points than their corresponding carbonylgruppenfreie connection.
The respective reactivity of the carbonyl compounds can be removed from a fixed number of carbonyl activity.
The following compounds are ordered according to increasing carbonyl activity. This can be derived from the M- and I- effects.
- Carboxylates
- Carboxylic acids
- Carboxamides
- Carboxylic acid esters
- Esters of thiocarboxylic
- Ketones
- Aldehydes
- Carboxylic
- Carbonyl halides
Reactions (selection)
Chemical conversions of carbonyl groups ( carbonyl ) are among the most common and important chemical reactions:
- Nucleophilic addition. Addition of hydrocyanic acid yields cyanohydrins
- To primary reduction (chemistry) or secondary alcohols
- Condensation reaction
- Michael addition of
- Enolate chemistry
- Alkylation by reaction with organometallic reagents (for example, Grignard reagents )
- Reaction with primary amines to form imines
- Reaction with hydroxylamine to form oximes
- Reaction with hydrazine to form hydrazones
- Reductive amination of primary or secondary amines formation.
Keto -enol tautomerism
An important aspect of the carbonyl is the so-called keto -enol tautomerism. Carbonyl compounds are in chemical equilibrium with its " enol " before, so far as they have alpha- hydrogen atoms in.
Examples of carbonyl compounds
Some examples of compounds with carbonyl groups ( class of substances, occurrence):
- Acetone ( a ketone, a solvent contained in the nail polish remover);
- Formaldehyde (aldehyde, a preservative for medical preparations );
- Acetic acid ( carboxylic acid contained in vinegar );
- Phosgene ( acid chloride of carbonic acid, a highly reactive product of the chemical industry);
- Carbonic acid ( dissolved carbon dioxide in the form of sparkling water);
- Glycine ( and all other amino acids, the building blocks of proteins);
- Polycarbonate ( polymers, a material made from the eg CDs ).
- Flavones ( flower pigments )