Chemical potential
The chemical potential is an intensive thermodynamic state variable, characterizes the ability of a substance,
- To react with other substances ( chemical reaction);
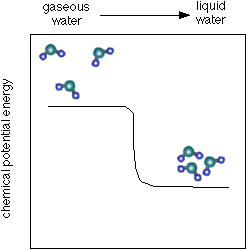
- In another state form move ( phase transition);
- To reallocate the space ( diffusion).
It is therefore suitable to describe all types of material reactions and reactions in which photons, phonons, electrons or holes are involved.
A reaction, conversion or redistribution can only take place voluntarily, when the chemical potential in the final state is smaller than the initial state:
The size μ, we call chemical potential today, by Josiah Willard Gibbs (1839-1903) was introduced, which she called " potential of a substance ," occasionally - to avoid confusion with other potentials - also called " intrinsic potential ".
( Unofficial ) A measure of the chemical potential is the " Gibbs " (= 1 J / mol), the unit of molar energy.
Definition
The chemical potential is defined by the fundamental equation of Gibbs internal energy U:
It is
- T is the absolute temperature
- S is the entropy
- P is the pressure
- V is the volume, and
- The amount of substance of a system component i
It follows that the chemical potential of the function U (S, V ) can be calculated:
The index indicates the to be kept constant sizes. Those are the amounts of all of the system components except
Alternative formulations
Alternatively, μ can also from the Legendre transform of U ( V, S, ) calculate, namely as:
- From the enthalpy H = U pV
- From the free energy F = U - TS
- From the Gibbs free energy G = U pV - TS
From the last equation it follows that the chemical potential of a substance whose partial molar free enthalpy corresponds ( Gibbs free energy).
Equilibrium conditions
In chemical equilibrium, the chemical potential of the reactants is equal to the product of:
Similar to the thermal equilibrium between the two systems, the temperatures are the same or electrical balance the electrical potentials.
With proper process control, the condition for chemical equilibrium can be formulated as a variational.
As in chemical reactions often pressure and temperature are kept constant, the minimum principle of the free enthalpy G is the criterion most commonly used. But the general balance criterion is the disappearance of the chemical potential difference, as it is irrespective of which other variables are held constant; it is also the case even if none of the other variables during the setting process of the equilibrium constant.
Interpretation
Indicates the dependence of the thermodynamic energy terms, and the composition of the system.
Each potential difference describes the system's ability to do work. Therefore, chemical reactions possess as long as a driving force ( " voluntariness of run " ) until an equilibrium is established by the chemical potentials of all the materials align ( The sum of the chemical potentials multiplied by the stoichiometric coefficient is zero, or in other words, the affinity is zero).
For pure substances ( component systems ), the chemical potential is constant and equal to the molar Gibbs energy. The molar Gibbs free energy for the formation of many substances under standard conditions are tabulated and allow the calculation of chemical potential differences in reaction mixtures. When given, and nj = 1k so that the calculation of the heat of reaction and the reaction direction is possible.
For crystalline solids corresponds to the chemical potential of the electrons in K of the Fermi energy.
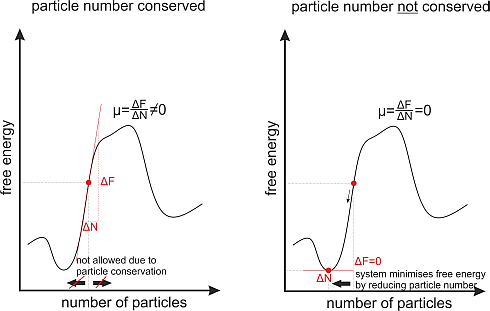
For quasi-particles, the chemical potential () vanishes, since there is no particle number here. Typical quasi-particles are, for example, Phonons or magnons, which can be described within the framework of quantum statistics.
Values
The values of the chemical potential are for standard conditions (T = 298.15 K, p = 101.325 kPa) tabulated below " Web Links ".
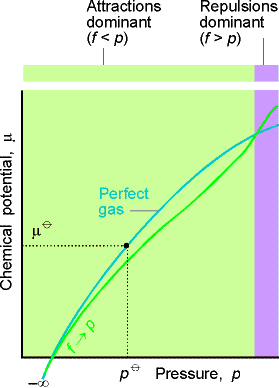
Is the chemical potential for a specific condition (eg standard conditions) is known, it can be used for pressures and temperatures in the vicinity of this state calculated in linear approximation:
With
- The temperature coefficient of
- The pressure coefficient
From the Maxwell relations it follows that
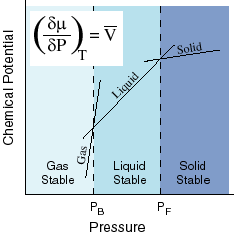
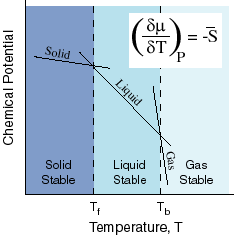
- The temperature coefficient of the negative molar entropy is equal to:
And
- The pressure coefficient is equal to the molar volume:
Concentration dependence
For the practical definition of the concentration dependence of the chemical potential is split into a concentration- independent and concentration-dependent summands:
With
- ° - chemical potential at standard pressure, which is still dependent on the temperature
- R - gas constant
- - Thermodynamic activity of component I, based on the standard activity ( the braces are to be understood as a mathematical operator that means ". Divide ai by the standard activity " )
The activity can often with sufficient accuracy by the concentration ci be replaced (for gases ) ( solute ) and the partial pressure pi in calculations. If it is in the system under consideration to an electrolyte, the Debye- Hückel theory provides an activity coefficient, with the help of which the concentration can convert in the activity.