Enthalpy
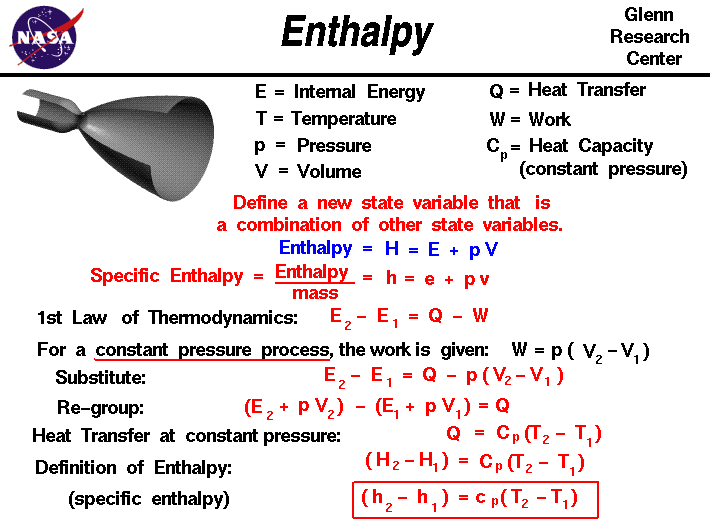
The enthalpy ( by ancient Greek ἐν en, ' and θάλπειν thálpein heating ', ' heat '), also known as heat content, is a measure of the energy of the thermodynamic system. It is usually indicated by the letter (unit: J) symbolizes, where the H is derived from the English heat content. And the specific enthalpy (unit: J / kg): In chemistry and technique also the molar enthalpy ( J / mol unit ) play an important role. They describe the enthalpy in terms of the amount of substance or mass, and therefore ( in contrast to the enthalpy) intensive quantities.
In other ( and in practice is much more common ) sense of the concept of enthalpy, however (see below) also used for enthalpy, ie increase or decrease of the energy content of a thermodynamic system, which is then used instead of the symbol the symbol.
The enthalpy is the Legendre transform internal energy with respect to the volume. She is the thermodynamic potential in the SPN ensemble and thus also an extensive state variable.
- 3.1 of vaporization / condensation enthalpy
- 3.2 sublimation
- 3.3 fusion / crystallization
- 3.4 lattice enthalpy 3.4.1 solvation, hydration enthalpy
- 3.4.2 enthalpy of solution / crystallization
- 4.1 of reaction
- 4.2 standard enthalpy
- 4.3 Advanced Usage
- 4.4 Technical applicability
Generally
The enthalpy is the sum of two parts, the internal energy and the volume of work:
The intrinsic energy from the thermal energy is - based on the omni-directional movement of molecules (kinetic energy, rotational energy, vibrational energy ) -, chemical bonding energy and the potential energy of atomic nuclei. In addition, interactions with electric and magnetic dipoles. It takes approximately proportional to the temperature of the system and is at absolute zero is equal to the zero-point energy.
The volume of work is clear in this case, the work has to be done against the pressure, to produce the volume occupied by the system in the considered state ().
Differentially expressed will of
( Note the difference between " steep" and italics differentials versus: the former should be reserved for state variables. )
Standard enthalpy
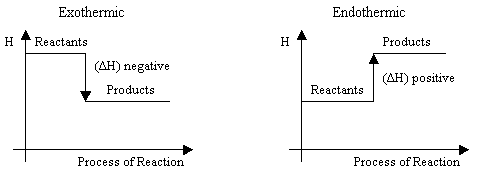
The standard enthalpy of formation is the energy that is released during the formation of one mole of a substance from the allotropisch most stable form of the pure elements under standard conditions ( 101,3 kPa and 25 ° C) (exothermic reaction, negative sign ) or the formation is required ( endothermic reaction, positive sign ). It is expressed in kilojoules per mole, and symbolically designated ( f engl of formation, education, . Exponent of zero stands for standard conditions).
If it is negative, as in the formation of the substance is free from the elements of energy, while if it is positive, the energy for formation of the substance must be expended from their output elements. Strongly negative values of the standard enthalpy of formation is a hallmark of most chemically stable compounds (ie, in their formation much energy is released and the destruction of the bonds a lot of energy has to be expended again). The standard enthalpy of formation of the chemical elements in their most stable state (H2, He, Li, ...) is fixed by definition at 0 kJ / mol.
An important application of standard formation is that so let enthalpies calculated by the set of Hess: So the reaction enthalpy of a given reaction under standard conditions is the difference between the standard enthalpies of the reaction products ("Products" ) on the one hand and the starting materials ( reactants; " reactants " ) on the other. Symbolically this can be represented by the following formula:
This is equivalent to the statement that the enthalpy of formation of a substance does not depend on the way of its production only from the substance itself, and under normal conditions. The enthalpy of formation is thus a thermodynamic state variable. All values refer to the thermodynamic equilibrium, otherwise the temperature would not be defined.
The standard enthalpy of formation may be indirect, with the help of the set of Hess, be determined from enthalpies of reactions in which the substance in participating as a reactant or product. May, if no experimental data are available to estimate the standard enthalpies also be estimated by group contribution methods. Is suitable for this especially the increment method to Benson.
Inorganic substances
Organic substances
Physical meaning of the enthalpy ( thermodynamics)
The thermodynamics describes in the strict sense only the intermolecular forces, ie the energetic relationships ( phase states and their changes ) between the individual molecules of a substance.
Enthalpy of vaporization / condensation enthalpy
The molar enthalpy of vaporisation is the energy required to transport one mole of a substance under isobaric and isothermal conditions from the liquid to the gaseous state.
The enthalpy of vaporization is the fabric and the boiling point dependent, being is always positive and its sign is therefore not specified in the rule.
Condensation enthalpy is therefore the energy that is released when one mole of a substance condenses, which goes back isothermal and isobaric from the gaseous to the liquid state. She always wears a negative sign.
In general, enthalpy of vaporization data are either based on 25 ° C or the corresponding boiling point in Table works, it is always.
For mixtures or solutions of substances, the enthalpies add in proportion to their mixing proportions.
No Verdampfungsenthalpiewerte are available If a substance can be this easy for any temperatures calculated from the vapor pressure curve according to the Clausius- Clapeyron.
In rare cases, values for heats of vaporization were tabulated. The enthalpy of vaporization can always be derived by forming the difference between the thermodynamic data when standard enthalpy values for the liquid and gaseous state are known, for example, water, carbon disulfide, methanol, ethanol, formic acid, acetic acid, bromine in the table above.
Note: The difference of the two values 170 and 204 is in the normal range. The enthalpy of vaporisation is dependent on the temperature decreases in the direction of the boiling point and is at a minimum when it reaches the critical temperature point.
Sublimation
Sublimation describes the transition of a solid to the gaseous phase while avoiding the liquid melt phase (technical application in the freeze-drying). The sublimation enthalpy is partially listed in tables. In principle, this may be combined with the same reference temperature and melting and vaporization enthalpy.
Sublimation enthalpy of fusion = enthalpy of vaporization.
The sublimation enthalpy can always be derived from the thermodynamic data when standard enthalpy values for the solid and gaseous state are known.
Note: This example shows that one can also calculate tasks in principle, which are practically impossible to carry out. The " sublimation of elemental carbon " has been " determined ".
Enthalpy of melting / crystallization
After the heating of a solid substance to their melting point temperature of heat of fusion is absorbed without the temperature continues to rise in this temperature. This form of heat is called the latent heat because it does not cause a change in temperature. For ionic solids formed during the phase transition solid / liquid salt melts easily with mobile ions (technical application in the fused salt electrolysis ). Salt melts at 800 ° C.
Enthalpies of fusion are rarely recorded in tables.
The heat of fusion can always be derived from the thermodynamic data when standard enthalpy values for the fixed and the liquid state are known.
When reversing this process, the crystallization from the melt, the ions of a salt can associate directly to their solid crystal lattice. While the resignation of sodium chloride crystals from the melt -25.2 kJ / mol enthalpy of crystallization released (29 ± 1 kJ / mol at 800 ° C or ).
Experience shows " supercooled molten salts " by a spontaneous onset of crystallization can release significant amounts of heat. (Application: heating pad ).
Lattice enthalpy
According to a common definition, the lattice energy is the energy that must be expended in a vacuum ( ie external pressure ), in order to convert a crystalline ionic solid into the gas phase (ie sublimation ), ie to separate it into gaseous ions. The lattice energy and lattice energy are qualitatively different. The lattice energy is an internal energy, while the lattice enthalpy is an enthalpy. Thus, the lattice enthalpy taken into account in addition the exact amount of volume work against a constant external pressure. If one has determined for the divergence Bring the ingredients of the solid molar lattice enthalpy, as is the molar lattice energy. This is based on the amount of change in volume.
The lattice enthalpy of NaCl is composed as follows:
Comparison: This is about twice the required energy that would be released in the highly exothermic reaction of sodium metal and chlorine gas. The formation of gaseous ions is therefore extremely endothermic.
The lattice enthalpy AH ° L depends on the size and charge of the ions involved and is always positive in this type of definition, since the grid would not be stable otherwise. The highest lattice energy has aluminum oxide Al2O3 ( Al3 and O2 - ) on with 15157 kJ / mol.
Frequently, the lattice energy of reaction is defined as the formation of the solid Salzgitter from ions in the gas phase. If the lattice energy is defined, the process is exothermic and the corresponding enthalpy change is negative specify. The lattice energy of aluminum oxide would then, for example -15 157 kJ / mol.
The lattice energy depends both on the size of the ions involved: The larger the ion, the smaller the vacant lattice energy because the forces of attraction with increasing distance of the positive nuclei decrease of the negative electron shell of the binding partner.
Examples: molar lattice enthalpy of the alkali metal fluorides at 25 ° C in kJ / mol:
On the other hand, the lattice energy depends on the electric charge of the ions involved from the greater the charge, the greater the attractive forces, and the larger the lattice energy.
Examples: molar lattice enthalpy at 25 ° C in kJ per mol ( in the examples changes the ionic radius only slightly ):
The highest molar lattice enthalpy has aluminum oxide Al2O3 ( Al3 and O2 - ) on with 15157 kJ / mol. This makes you look at the processes of the so-called aluminothermy advantage. The resultant alumina in the highly exothermic heat forming can be used, for example welding.
A similar effect in graphite under neutron irradiation see Wigner energy.
Solvation, hydration enthalpy
It indicates how much energy is released when gaseous ions attach to solvent, thus form solvated ions. For the most frequent case, solvent = water is called hydration enthalpy.
The enthalpy of hydration of gaseous ions of sodium chloride is -774 kJ / mol in total strongly exothermic.
Enthalpy of solution / crystallization
The enthalpy of solution of salts include 1) the separation of the ion - grating in single - ion, and 2) the solvation of the individual ions. Part of step 1) is highly endothermic, part of step 2) very strongly exothermic.
Enthalpy of solution = lattice enthalpy of solvation.
Enthalpy of solution of NaCl in water = ( 778 kJ / mol ) ( -851 77 kJ / mol ) = 4 kJ / mol ( 25 ° C).
This value is in good agreement with table works 3.89 kJ / mol for the brine heat. When loosening thus occurs a very slight cooling of the solution.
Of course you do not go for practical calculations of the heat of solution going through the lattice energy, but is expected directly and with just a few table values (sometimes one finds the value ( NaCl) hydrate = -407 kJ / mol instead of the individual ions. ):
The solvation can always be derived from the thermodynamic data, if you find standard enthalpy values for the solid and solution state of aggregation, such as formic acid and carbon dioxide in the table above. It applies to " infinite dilution ".
By reversing this process, the crystallization from solution, give the dissolved ions of a salt 1 ) from its solvation shell and 2) close in a solid crystal lattice. While the resignation of sodium chloride crystals from water -3.89 kJ / mol enthalpy of crystallization released.
Intermolecular enthalpy contributions
Varying levels of interactions between the molecules are the reason that substance groups have similar or very different sublimation.
- Weak contributions provide London forces, dipole- dipole interactions and ion-dipole interactions with 1-15 kJ / mol binding. They are used as van der Waals interaction combined. See as examples the melting and vaporization of hydrogen, carbon monoxide and methane.
- Stronger contributions provide hydrogen bridge bonds with 20-40 kJ / mol bond (depending on polarization). See as examples the melting and vaporization of water, methanol and formic acid and Hydratationsenthalpien. Hydrogen- bonds are also responsible for ensuring that the boiling point of water at 100 ° C, while that of hydrogen sulfide only -83 ° C ( see boiling point anomaly ).
- Very strong contributions provide ion-ion interactions in crystals. Sodium chloride is not in the crystal consists of discrete molecules of NaCl, but from an equal number of sodium cations and chloride anions, which have arranged exactly in the crystal lattice according to the Coulomb forces.
Chemical significance of enthalpy ( thermochemistry )
The thermochemistry describes in the strict sense only the intramolecular forces, ie the energetic relationships between the individual atoms of a molecule. Covalent bonds contain about 150-1000 kJ / mol binding energy, ionic bonds approximately five times as large amounts.
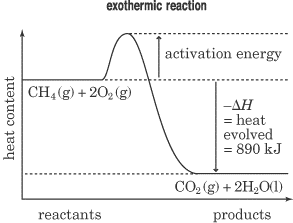
With knowledge of the standard enthalpies of reactants and products of a possible chemical reaction energy can be accounted for gross. The most important issue is often whether a process is exothermic or endothermic, and how much.
With details such as evaporation, melting, solvation or Kristallisationsenthalpien partial steps can within the chem. Response to clarify energetically.
Enthalpy of reaction
The reaction enthalpy is the energy that is released or needed if between the molecules of two substances, new chemical bonds are formed. It depends on the reaction partners ( starting materials) and the type of chemical bond in the product. For calculating comparing the sum of the enthalpies of the products of the starting materials. The difference is the reaction enthalpy, which can then be standardized by reference to the number of moles of each product of interest:
Standard enthalpy
The combustion is a chemical reaction. The reaction enthalpy of the combustion reaction and the standard enthalpy of a substance is the enthalpy change that occurs when a substance under excess O2 (O2 pressure ) and standard conditions ( 101.325 kPa and 25 ° C) to completely burn. By definition, this heat of combustion refers to the formation of gaseous carbon dioxide and liquid water (or N2) as end products; under oxygen pressure can not form gaseous water. It is called with ΔVH ° and is identical to the condensing ms
In an autoclave tube following reaction is performed with an oxygen pressure:
The same reaction in an open burner flame; it arise only gaseous combustion products:
Advanced Usage
It is idle for any reaction the standard enthalpies of reactants and products gather up, also still in the correct state of aggregation. In addition encountered with larger molecules quickly in a "data vacuum ". The following simplified considerations have been proven in practice:
Therefore, one can calculate with the help of numerous tabulated increments for gaseous molecules to Benson normal organic chemical reactions such as halogen additions, cycloadditions, esterification with acids or anhydrides, hydrolysis etc..
In the following example the reaction power of the bromine addition is calculated on the ethylene with Benson increments and estimated for comparison of binding energy of involved bonds. (Note: bond dissociation energies are averaged, no standard formation! )
Addition of bromine to an alkene, reaction enthalpy calculated with standard formation:
Addition of bromine to an alkene, reaction enthalpy calculated with increments to Benson:
Addition of bromine to an alkene, reaction enthalpy estimated with binding energies:
The best estimate for reaction enthalpies achieved with standard formation or increments to Benson, when using " binding energies " the uncertainty of ± 200 kJ / mol is much too high.
Industrial Applicability
The reaction enthalpies GLars organic reactions are in the range -160 to 100 kJ per mol " reactive centers ". As a very strong exothermic all addition reactions prove with epoxides, anhydrides, and halogens. This thermochemical considerations to make any statement about how fast these heats of reaction are released. You only make the statement that by the end of the reaction heat is released. Each reaction to increase their speed two to three times in case of temperature increase of 10 K. Conversely, a two-fold dilution of the reactants, often a halving of the rate of reaction or the heat capacity of the reaction. Calculated reaction enthalpies are used to calculate in a system of reactants and solvents on the heat capacities of the temperature curve. Large-scale systems have limited cooling capacity ( -Wärme/Zeit ), this is often not taken into account in the laboratory.
Binding energy / dissociation
The binding energy or binding strength indicates the " stability" of the atomic bond. The provision is directly possible only with diatomic symmetric molecules such as hydrogen or halogens. In these cases, the dissociation energy for the formation of two identical radicals can be easily measured / calculated. In " element radicals " refers to the standard formation of radicals as Atomisierungsenthalpien.
In all other cases, values for the " binding energy " are only indirectly by comparing multiple dissociation energy measurements on homologous molecules. The values vary depending on the substitution pattern at the radical centers.
The standard enthalpy of formation of gaseous bromine radicals includes the enthalpy of vaporization (31 kJ / mol), which is necessary in order to transport the liquid bromine in the gas phase. The standard enthalpy of formation of gaseous IOP radicals includes sublimation (62 kJ / mol), which is necessary in order to transport crystalline iodine in the gas phase. The standard enthalpy of formation of gaseous CARBON radical is identical to the standard formation of gaseous carbon vapor.
Joule-Thomson effect ( production of low temperatures )
Physically, as to the amount of substance molar enthalpy of technical importance.
Unlike other sizes in the Joule- Thomson effect is the enthalpy of a fluid as it flows through the system by a throttle valve (ie a very strong restriction for pressure decrease) constant, although prevail on both sides of the valve different pressures.
This fact and the Joule -Thomson effect can be technically used for the liquefaction of gases by the Linde process.