Mesomeric effect
By a substitution reaction of molecules containing a resonance, it may happen that the substituent may take part in the resonance. Thus, the mesomeric system is increased. In this case, the first deputy has a mesomeric effect (short: M- effect). Mesomeric effects affect any other reaction on the molecule in question. You determine at which position - will likely be a further reaction to proceed - ie to which atom. In addition, several resonance structures, so that the molecule is stabilized result.
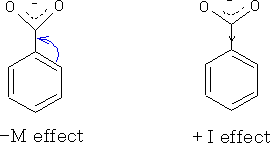
The effect of ion impact on reaction rates and equilibria, since the p-type or π - orbitals of substituents overlap with the rest of the molecule. It comes to the delocalization or the extension thereof. The effect will be abbreviated with m. In addition, there is the inductive effect ( -I and I effect).
In the electrophilic second substitution on the benzene ring conduct ( = determined) the Erstsubstituenten the position of the other. In general we can say that Erstsubstituenten that have a M effect direct ortho / para position, those with - M effect in the meta position. This can be explained by the stability of the transition states occurring during the substitution.
- 2.1 substituents with - M effect
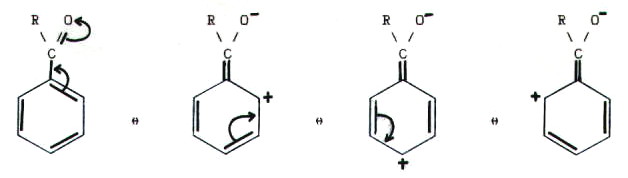
M effect
The substituent has a free electron pair that it can provide for the mesomerism available. For M - effects, the electron density of the mesomeric system increases. A possible electrophilic second substitution is favored by the M - effect is thus activating in terms of reactivity. With halogens as Erstsubstituenten the substitution rate is, however, due to the overall reduced -I effect, but is M- effect the more in the ortho / para -position conducted ( the case of aromatic systems).
For example, reaction of bromine and benzene: benzene containing a mesomeric system and bromine has three lone pairs. By the substitution of bromine is bonded with a single bond to a carbon atom of the benzene. A lone pair of electrons of the bromine may be involved in the resonance, resulting in new mesomeric structures.
Substituents with M effect
- -O-
- - NH2
- - NR2
- -OH
- OR
- = -NHC (O) R
- = -OC (O) R
- - (Aryl) (e.g., phenyl group)
- - CH3
- -Br,- Cl, -I,- F
- M effect
Wherein -M effects the mesomeric system electron density is removed, as the substituent having a double or triple bond. This complicates further substitutions or sets the activation energy, which is necessary to, up. The - M effect thus acts from deactivating reaction kinetic point of view. In addition, the following groups act meta- directing (with aromatic systems ).
Substituents with - M effect
- - COOR
- -COOH
- -CHO
- = -C (O) R
- -CN
- -CH = CH- COOH
- - NO2
- -SO3H
Acid strength
Substituents with -M effects cause an increase in the acid strength of the aromatic, acidic compounds as they stabilize the resulting anion of the acid. Nitrophenols are stronger acids compared to phenol ( pKa = 9.99). Trinitrophenol reached a pKa of 0.29.
Base strength
With an aromatic ring to which an amino group is, electrons are pushed to the ring due to the M effect. Thus, the functional group is positive, the pKb value increases in total for this connection. That is, the " base strength " decreases.

_V.1.png)






.png)