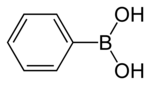
Phenylboronic acid
- Dihydroxy (phenyl ) boron
- Phenylboronic
Colorless to pale yellow odorless solid
216-219 ° C
8.83
Soluble in water (10 g · l-1 at 20 ° C)
Attention
740 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
Benzene boronic acid, also known as phenylboronic acid, is a chemical compound selected from the group of the organoboronic acids.
Production and representation
There are numerous methods to synthesize benzene boronic acid. A common synthesis using phenylmagnesium bromide and trimethyl borate to form the corresponding ester which is then converted by hydrolysis into phenylboronic acid.
An analogous synthesis can also be effected starting from phenyllithium.
Properties
Benzene boronic acid is a colorless to pale yellow odorless solid. By dry heating the compound can be trimerizes elimination of water to the anhydrous Triphenylboroxin.
Use
Benzene boronic acid is used in many cross - coupling reactions. In 1979 Miyarura and Suzuki found a carbon-carbon bond formation reaction (today as Suzuki coupling called ) which aryl boronic acids with aromatic halogen compounds under catalytic use of palladium - phosphine complexes convert to biphenyl derivatives or vinyl aromatics.