Radical initiator
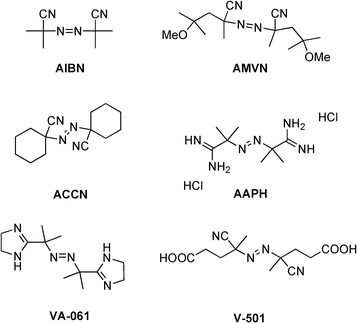
Free-radical initiator is the name for a particular group of chemical compounds which are useful for the chemical synthesis. There are initiators of radical reactions (see also: Chemical reaction ) incurred by thermolytic cleavage ( starting at room temperature) or photolytic (<300 nm). So make those reactive radicals, which "Start ", select the radical reaction (eg polymerization). Preferred initiators are azo compounds and peroxides. The radical initiator has an azo group (-N = N- ), as this is also known as azo initiators.
Well-known examples are azobisisobutyronitrile ( AIBN short ) and dibenzoyl peroxide ( BPO ):
- AIBN decomposes into two isobutyronitrile radicals and a nitrogen molecule.
- DBPO divided into two benzoyloxy radicals.
The cleavage is always at a heteroatom - heteroatom bond or hetero atom or metal -carbon bond, never at a CC bond. Free-radical initiators useful in the preparation of many polymers ( such as polyvinyl chloride or polystyrene) is indispensable.