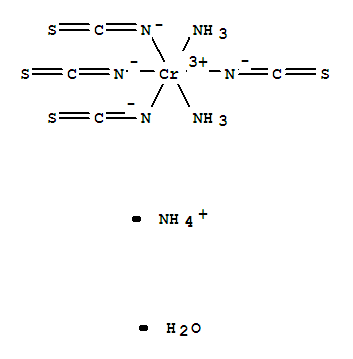
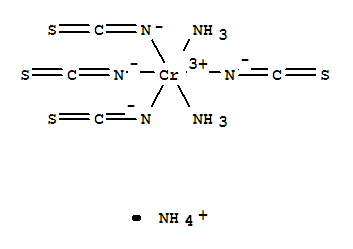Reinecke's salt
Ammoniumtetrathiocyanatodiamminchromat (III)
Dark red crystals
- 336.43 g.mol -1
- 354.42 g · mol -1 ( hydrate)
Fixed
268-272 ° C ( decomposition)
Soluble in hot water and ethanol
Attention
Template: Infobox chemical / molecular formula search available
The Reinecke salt is a complex compound of trivalent chromium with the constitutional formula NH4 [ Cr ( SCN ) 4 ( NH3) 2]. It forms dark red crystals which are soluble in hot water and ethanol. The salt was first prepared in 1863 by Albert Reinecke from Detmold by fusing ammonium thiocyanate and potassium dichromate.
Properties
The salt consisting of an ammonium ion and a complex anion, which is made of a Cr 3 ion as a central atom and six ligands. The complex is octahedrally coordinated.
Use
The Reinecke salt is used in the analysis for the detection of cations. For example, coincides with mercury (II ) ions, a poorly soluble bright red precipitate of so-called mercury (II) Reineckat from:
Copper ( I) ions form a yellow precipitate of low solubility:
The proof with Reinecke salt is very sensitive. This allows to use also for quantitative determination of the photometry this method. If you, for example, mercury (II) in the presence of thiourea Reineckat, the result is a complex, which is soluble in ketones and can photometry at 520-540 nm.
Meanwhile, in addition to inorganic cations, a variety of reactions with organic cations known, in particular with ammonium ions from primary and secondary amines.