Soil pH
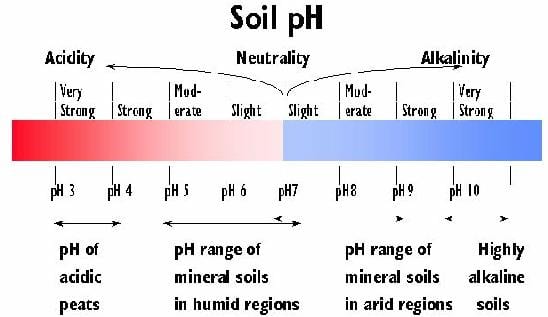
The soil pH is a feature of the acid and base content of the soil. It is based on the measure of the pH value and is influenced by the natural buffering system. The soil pH value is ( n, calcium chloride or potassium chloride 0.01 ) was measured in a suspension of soil in neutral salt solutions by reaction electrometrically.
Here, because the sorbed to the exchangers H ions are displaced by the CaCl2 ions, the so- measured pH of about 0.3 to 1.0 unit lower than the pH value, measured in aqueous suspensions, but are not all data in the literature corrected accordingly.
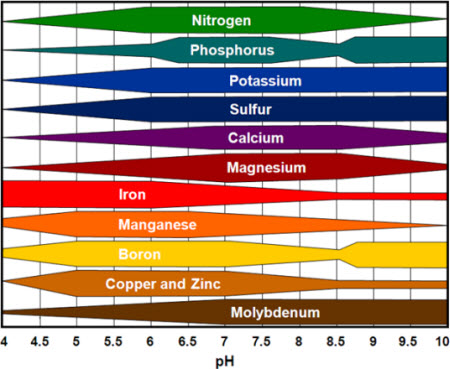
Availability of nutrients depending on soil pH
The table shows the areas of high availability of various nutrient salts as a function of pH:
The optimum pH value of the soil is affected by plant species, soil organic matter (humus, etc.) and clay minerals. The following pH values are recommended for typical agricultural use:
- 5.0 to 5.5 at < 4% organic matter and <5% clay content
- 5.4 to 6.0 at from 5 to 12 % clay
- 6.0 to 6.5 at> 13% clay content
Particularly critical are very small pH values to be considered (strongly acidic environment ), as toxic concentrations can then be released from aluminum. Very basic soils can suffer from manganese deficiency. Generally the pH increase results in a structure improvement.
The majority of crops preferably a neutral or slightly acidic soil, since the solubility of most of the nutrients for healthy growth of the plants at a pH is 6.3 to 6.8 at the highest. However, some plants prefer acidic (for example, potatoes, strawberries ) or more alkaline (eg, carbon ) ratios. Can be partially attenuated by buffering functions of the soil acidic soil entry through precipitation or emissions.