Sucrose
Monoclinic - sphenoidisch
- Sucrose
- α -D-glucopyranosyl - β -D - fructofuranoside
- β -D - fructofuranosyl - α -D-glucopyranoside
- Granulated sugar
- Cane sugar
- Beet sugar
- Table sugar
Colorless and odorless, crystalline solid with a sweet taste
Fixed
1.57 g · cm -3 ( 30 ° C)
185-186 ° C (dec. from 160 ° C)
Very well in water (1970 g · l-1 at 20 ° C)
29.7 g · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
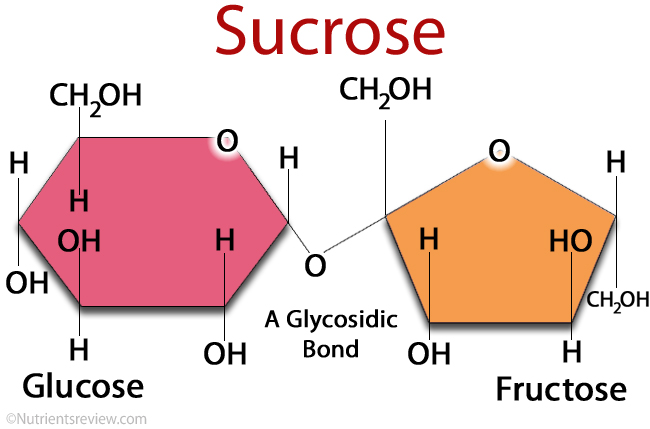
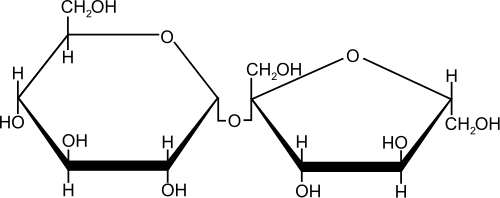
Sucrose [ zaxaro zə ː ], also sucrose, is the household or crystal sugar, which is commonly used as a " sugar ". Both the sugar and the sugar cane and palm sugar containing this disaccharide in economically viable quantities. In a sucrose molecule α -D-glucose and β -D-fructose via a α, β -1 ,2- glycosidic bond are each connected.
- 3.1 Chemical Properties
- 3.2 Physical Properties 3.2.1 heat and burn
- 3.2.2 Water
- 3.2.3 rotation of polarized light
History
The sakcharon the ancient world (from Sanskrit carkara " fragile ", "stone- like") was originally the Tabaxir (bamboo stone), the healing powers were attributed. Only later the Arabs rendering the word on the similar-looking cane sugar. Even the word origin for sugar from śárkara (Sanskrit for " sand ", " gravel " ) can be found in the literature. After Lippmann cane sugar was known in India only in the third to the sixth century.
The constitution was elucidated by Walter Norman Haworth.
Occurrence, extraction and importance in plant
Sucrose is made up of many plants by photosynthesis, for the extraction of table sugar are mainly sugar beet, sugar cane and palm sugar (mainly in Indonesia ) is important. In smaller amounts of sucrose is also obtained from the sap of the sugar maple. In addition, the containing exclusively or predominantly sucrose phloem sap of many plants is the basis for honey production - by the bees either directly plant secretions such as nectar or the honeydew called exudates of phloem sap sucking insects (especially Schnabelkerfen such as aphids, scale insects, psyllids, moth scale insects and various cicadas ) collect.
Biosynthesis
The biosynthesis of sucrose takes place in the cytoplasm of plant cells of the hexose intermediates UDP -glucose and fructose 6 -phosphate. The two monosaccharides are formed from triose phosphates, which arise as a net gain in carbon assimilation of photosynthesis ( Calvin cycle ) in the chloroplast. The two triose phosphates glyceraldehyde -3 -phosphate and dihydroxyacetone phosphate are used either in the chloroplast for the synthesis of starch ( storage starch ) or exported from the chloroplast into the cytosol, where it caused hexoses serve the synthesis of sucrose ( or other carbohydrates or amino acids).
To fructose -1 ,6- bisphosphate is first formed by a condensation reaction between glyceraldehyde -3-phosphate and dihydroxyacetone phosphate, which is then reacted by dephosphorylation to fructose-6 -P. Fructose -6-P also glucose -6-P is formed by isomerization, by the subsequent reaction (to be Transisomerisation to glucose -1-phosphate ) and uridine triphosphate (UTP ) is activated to uridine diphosphate -glucose (UDP -glucose ). Following the condensation of UDP -glucose and fructose 6- P to sucrose -6-phosphate is catalyzed by the enzyme sucrose -phosphate synthase. The energy needed for this brings the elimination of uridine diphosphate ( UDP). Finally, the phosphate group is cleaved in an irreversible reaction by the enzyme sucrose -phosphate phosphatase, so that sucrose is produced.
Importance as a transport sugar
Sucrose is the major transport sugar in plants. For this purpose, it is better suited as free hexoses, as it is chemically inert as a non- reducing disaccharide. The force generated by photosynthesis in green plants cells in light sucrose passes through passive transport into the apoplast and then by active transport in the phloem of the plant vascular tissue assimilatleitende. Phloem it is to other, non- photosynthetic tissues, such as growth sites or storage tissues, transported.
Other transport sugar are in some plant families (eg, cucurbits, walnut family ) Raffinosen.
Dismantling and recycling
For the sucrose degradation in the target tissues, there are different possibilities.
In growth areas such as shoot and root tips ( meristems ) is transported sucrose from the phloem through plasmodesmata symplasmatisch. In the cells it is cleaved in a reverse of the synthesis reaction by the enzyme sucrose synthase with UDP to UDP - glucose and fructose. The two hexoses may converted to glucose -6-P and be introduced, for example, to produce energy in the glycolysis.
In storage tissues sucrose is transported apoplastic from the phloem to the target cells. It can be absorbed by active transport into the cell, where they are degraded by the sucrose synthase. The majority, however, divided on the cell wall of invertase to glucose and fructose. The two monosaccharides may be received by symporter of the cell, where they are transported as glucose -6-P in the chloroplasts and storage starch used for the synthesis of.
Properties
Chemical Properties
The sucrose as part of other sugars to carbohydrates. It is a disaccharide ( double sugar ). Sucrose consists of a dimer of one molecule of α -D-glucose ( pyranose form ) and β -D-fructose ( furanose ). These two molecules via a α, β -1 ,2- glycosidic bond linked ( α1 -2 glucose fructose ) located under the outlet of a water molecule (condensation reaction) is formed with each of the OH groups of the anomeric carbon atoms.
Sucrose is a non-reducing disaccharide. Non-reducing disaccharides are O -glycosidically linked via their anomeric two carbon atoms, their chemical name ends with SID. This means that in the sucrose molecule, the two components are connected together so that no aldehyde group by ring opening can be (either from glucose or fructose by the molecule ) is formed. These non- reducing atomic groups called acetals. Acetals are unlike hemiacetals relatively stable in basic and neutral media. They can only be opened by acid catalysis, wherein the disaccharide is cleaved into monosaccharides, in part, it arises invert ( material volume equal parts glucose and fructose). Sucrose shows almost no mutarotation due to the under remaining in neutral medium ring opening.
Physical Properties
Heating and combustion
Upon heating of sucrose at 185 ° C it melts and forms a brown decomposition expectant melt ( caramel ). If the sucrose combusted, there sugar charcoal and a foul-smelling gas.
Water
Sucrose is very soluble in water. The solubility is, as with most solids, temperature-dependent:
At 20 ° C., a 66 % solution (density 1.33 kg / l) at 100 ° C, however, a 84 % saturated solution (density 1.44 kg / l) who, by no more crystals on cooling, however, ( " hindered crystallization ").
Rotation of polarized light
Sucrose is chiral and therefore optically active: In aqueous solution, sucrose rotates polarized light clockwise (specific rotation angle = 66.5 °). By cleavage, a mixture of sucrose ( invert sugar) which is half of glucose and fructose from half. The mixture turns counterclockwise polarized light (specific rotation angle = -20 ° ), that is observed an inversion of the rotation direction ( " Inversion "); the 1:1 mixture of fructose and glucose is therefore also referred to as invert sugar.
Sweetening power
The sweetening power is a dimensionless quantity that indicates the relative sweetness of a substance. The values of the sweetening power relate to sucrose, which is assigned to a sweetening power of 1. The sweetening power is a semi- quantitative comparison especially to other natural or artificial sweeteners. Sweeteners may have a several hundred or thousand times sweeter compared to sucrose. Interestingly, part of a derivative of sucrose, D-( )- sucrose octaacetate to the most bitter compounds known.
Effect of sugar on the body
Until the Industrial Revolution in the 19th century pure sugar broad strata of the population was barely accessible in Central Europe. Sugar was supplied to the body mainly in the enjoyment of vegetables and fruit and honey. Only since the breeding of sugar beet in 1800 and the beginning of the industrial refining of sugar the body with large amounts of granulated sugar was confronted.
High sugar consumption, especially when it, for example, are " free" sugar from sweet drinks can lead to obesity and thus to an increased disease risk for diabetes mellitus.
Studies by John Yudkin suggest that there is a relationship between sugar intake and the incidence of myocardial infarction.
Missing or inadequate dental care after the consumption of sugary foods leads to the formation of dental caries. Many sugars can be converted to cariogenic acids by bacteria in the mouth. In particular, sucrose is processed by the bacterium Streptococcus mutans to dextran, with the help of these measures may be particularly stubborn cling to teeth.
The World Health Organization recommends that sugar should account for no more than 10 % of the human daily energy intake. This is exceeded in most industrialized countries.