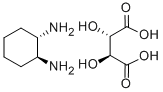
Trans-1,2-Diaminocyclohexane
- 694-83-7 (mixture of isomers )
- 1121-22-8 (trans- isomer )
- 1436-59-5 (cis- isomers)
- 20439-47-8 [ (1R, 2R ) - (- )-enantiomer ]
- 21436-03-3 [( 1S, 2S ) - ( ) enantiomer ]
Colorless liquid with an amine-like odor
Liquid
0.95 g · cm -3
183 ° C
11.5 hPa ( 70 ° C)
Soluble in water
Risk
Template: Infobox chemical / molecular formula search available
1,2- diaminocyclohexane is a chemical compound. It consists of a cyclohexane ring as a basic structure, are bonded to the two amino groups on adjacent carbon atoms and thus belongs to the group of diamines.
Isomerism
1,2- diaminocyclohexane, contains two stereogenic centers, therefore there are three stereoisomers: (R, R ) -1,2- diaminocyclohexane, and the mirror-image to (S, S ) -1,2- diaminocyclohexane as well as meso -1 ,2- diaminocyclohexane.
Representation
1,2- diaminocyclohexane may be prepared by a Curtius degradation of 1,2- cyclohexanedicarboxylic acid. Is trans -1 ,2 -cyclohexane dicarboxylic acid used as starting material, the amino groups in the product are also arranged in the trans position. Starting from cis-1 ,2 -cyclohexanedicarboxylic acid ( meso -1 ,2 -cyclohexane dicarboxylic acid ) is obtained analogously to cis-1 ,2 -diaminocyclohexane ( meso -1 ,2- diaminocyclohexane ). The resolution of trans -1 ,2 -diaminocyclohexane [ 1:1 mixture of (R, R ) -1,2 -diaminocyclohexane and (S, S) -1,2- diaminocyclohexane ] leaves into its enantiomers by diastereomeric salts treatment with enantiomerically pure tartaric acid accomplish.
Properties
It is a colorless liquid compound at room temperature, and boils at 183 ° C. The optical rotation of the pure substance at 55 ° C and a wavelength of 589 nm is -36 ° (R, R - enantiomer).
Use
By condensation reactions with α, β -diketones, 1,2 -diaminocyclohexane used in the synthesis of pyrazines. Under elimination of two water molecules diimines be first obtained, which can then be oxidized to form pyrazines.
By condensation with activated carboxylic acids ( Steglich esterification ) or carboxylic acid chlorides, amides can be prepared. This reaction is for example used for the synthesis of the Trost ligand.
Furthermore, 1,2- diaminocyclohexane are used as Diaminoligand in metal complexes. Here it occurs as a bidentate chelating ligand. For example, Oxaliplatinverbindungen used as cytostatic agents, the platinum complexes of 1,2- diaminocyclohexane.
Condensed 1,2- diaminocyclohexane, also with two equivalents of salicylaldehyde derivative to a salen derivative. These tetradentate chelating ligands form with cobalt ( II) complexes, which are used as oxygen transporters example, in the Jacobsen epoxidation use.