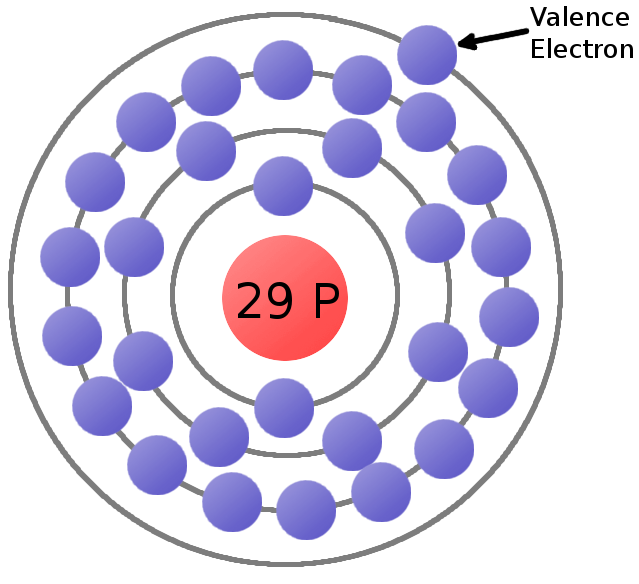
Valence electron
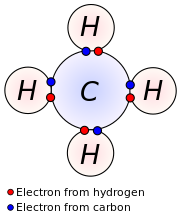
Valence electrons (often called outer electrons ), the electrons present in the outermost atomic orbitals and on bonds ( " valence " ) can participate between atoms.
In the main-group elements are the electrons in the outer shell ( valence ) in s -and p- orbitals, wherein the transition metals are on the outermost shell and the additional electrons in the d orbital of the preceding shell.
Are valence electrons involved in bonds, as they are thus bonding electrons. But they can also be non-binding and are then no bonding electrons. That is, the number of valence electrons true - depending on the chemical state of the corresponding atom - not always with the number of electrons involved in bonds actually match.
So, for example, the element has seven valence chlorine, the ( HClO4 ) are involved in bonds in the molecule of perchloric all. In contrast, in hydrogen chloride ( HCl) is involved in only one of the seven electrons to the electron pair bond.