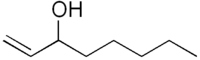
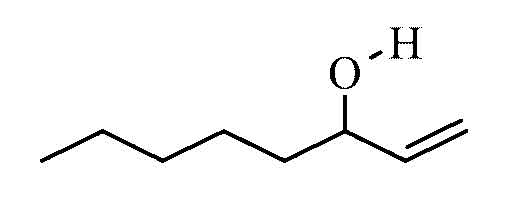
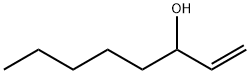
1-Octen-3-ol
- Vinylhexanol
- Amylvinylcarbinol
- Matsutake alcohol
- 3391-86-4 (racemate )
- 3687-48-7 [(R ) - (- )-enantiomer ]
- 24587-53-9 [( S) - ( ) enantiomer ]
Colorless liquid
Liquid
0.84 g · cm -3 ( 20 ° C)
-49 ° C
175 ° C.
1 hPa (20 ° C)
Poorly in water
1.4391 ( 12 ° C)
Attention
Template: Infobox chemical / molecular formula search available
1- octen-3 -ol ( octenol short ) is a chemical connection. In its pure state it is a colorless liquid with a musty smell that is hard flammable and can form explosive mixtures with air. It belongs to the alcohols, especially to the allylic alcohols, and is widely used as a metabolic product in nature. It is used as an attractant for mosquitoes and as a perfume ingredient. Of the two stereoisomers of this, only the (R ) - (- )-1- octen-3 -ol effect. Usually, however, the technically easier synthesizable racemate is employed. The taste of Octenols is perceptible even to the ppb range in foods.
Occurrence
Octenol is the taste -determining substance in many edible mushrooms. In particular, it arises at the moment of Anschneidens of mushroom flesh.
It is the main component in the essential oil of Rhododendron and determines the taste of the potato tuber. Included in no small amounts, it is still in wild bergamot, oregano and West Indian bay. It is effective against infection by molds such as Penicillium or Aspergillus and probably produced for this reason, as a secondary metabolite of fungi and plants.
Octenol is a metabolic product of many microorganisms, such as the fungal Penicillium. It is one of the substances that cause the musty smell moldy flats and remains a long time after the mold control perceptible. Also moldy vegetables and old books owe their flavor partially the octenol.
Octenol is partly responsible for many food flavors such as the smell and taste of blue cheese and camembert or Korkton of wine.
Role in insect metabolism
Octenol is next to 4-hydroxy -4-methyl -2-pentanone, 1-heptene -3-one and 3- octanone one of those odorous substances which, starting from plants and fungi, insects attract females and induce them to lay their eggs. 40 percent of the olfactory cells of tsetse flies respond to octenol.
Production and biosynthesis
Chemically preparing octenol two paths available:
- By Grignard reaction of acrolein with Amyljodid in 65 percent yield
- By selective reduction of 1-octene -3-one in 90 percent yield
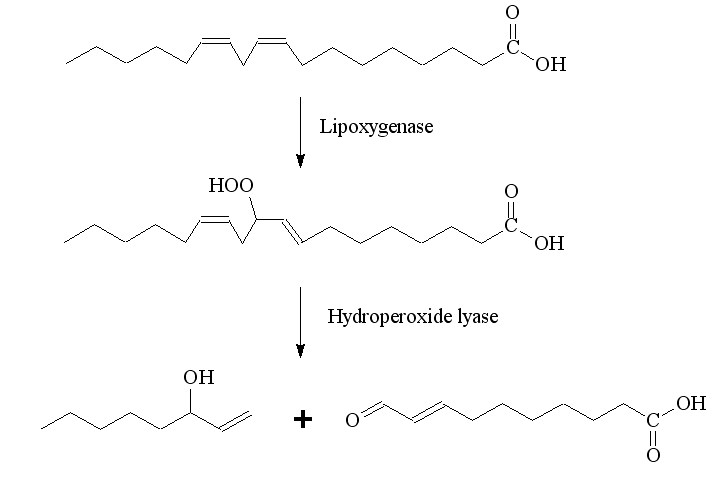
Biochemically (R ) - ( -)- octenol by peroxidation of linoleic acid, catalyzed by a lipoxygenase, and subsequent cleavage of the hydroperoxide, using a hydroperoxide formed. This reaction also takes place in the cheese and biotechnology for the production of ( R) - ( -) enantiomer used.
Use
Octenol is used as an attractant for tsetse flies, gnats and mosquitoes in professional attractant traps. In some perfumes it is contained up to 1 percent.
Dangerousness
Octenoldämpfe were classified as non-toxic; for swallowed octenol exists an LD50 of 340 mg / kg and on the skin, a LD50 of 3300 mg / kg. It is in large quantities as an eye irritant.
Imamdar and coworkers were able to establish in Drosophila and human cell lines, a disorder of the dopamine balance of 1- octen-3 -ol. Thus, the non-toxicity of Octenoldämpfen is questioned. Exhalations of octenol in moldy rooms could be responsible for neuropsychological problems and movement disorders. Octenol could be a factor for the Parkinson's disease.