2,4-Dinitrophenylhydrazine
- 2,4 - DNPH
- DNPH reagent
- DNP
Red solid
Fixed
0.84 g · cm -3
200 ° C ( decomposition)
- Almost insoluble in water and ethanol
- Soluble in dilute mineral acids and glacial acetic acid
Risk
> 500 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
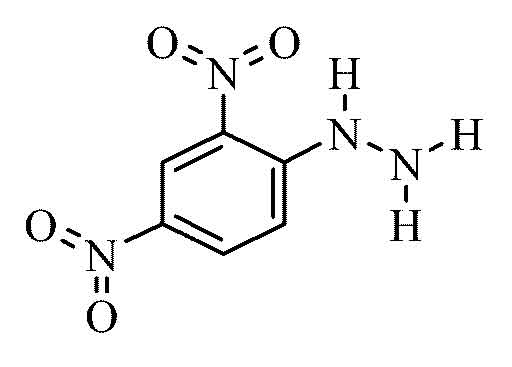
2,4- dinitrophenylhydrazine is an aromatic nitro compound with the formula C6H6N4O4.
Production and representation
The display may be effected by reaction of 2,4 -dinitrochlorobenzene with hydrazine.
Properties
The connection itself is a red -orange, odorless solid. Dry 2,4 - dinitrophenylhydrazine is explosive; in the decomposition of nitrous gases. It is stabilized with water, ie the commercially available product is about 0.5 ml of water per gram of DNPH was added to make the substance stored and transported.
Use
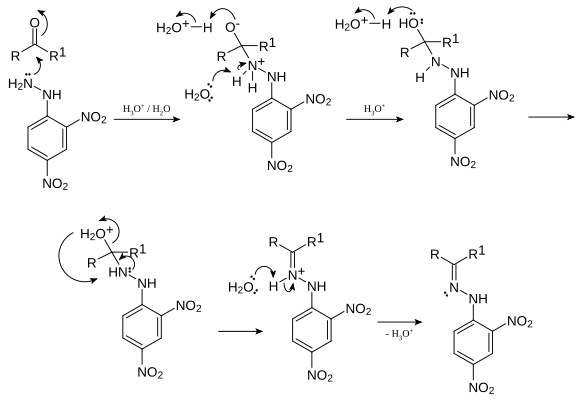
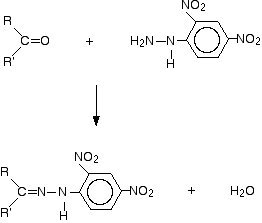
2,4- dinitrophenyl used as a detection reagent for the carbonyl group, a functional group in the organic- chemical compounds. The reaction is specific to carbonyl groups of aldehydes and ketones with carboxylic acids, amides or esters, however, no reaction takes place. The first step is a nucleophilic addition of the NH2 group to the carbonyl group, followed by a molecule is split off water. In this condensation reaction, reacting the aldehydes and ketones to the corresponding 2,4- dinitrophenylhydrazone (C = N- NR2). Mechanistically, this reaction corresponds to the formation of imines from aldehydes or ketones and primary amines.
If there is positive in the implementation of the test compound with 2,4- dinitrophenylhydrazine under slightly acidic conditions to form a precipitate which was the sample. Hydrazones shown have different, but sharp melting points, which makes possible the identification of the hydrazone (and thus of the starting material ) with a melting point measurement and comparison with tabulated values .