Anomeric effect
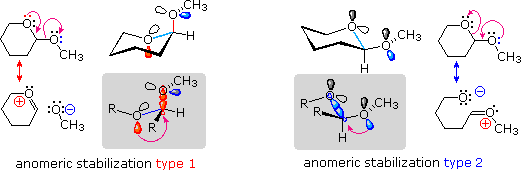
In the organic chemistry of the anomeric effect refers to the tendency of a heteroatom, which is substituted on a carbon atom adjacent to a hetero atom in the cyclohexane ring, ( sterically hindered less!) The axial position with respect to the preferred equatorial position, steric considerations contrary. In other words, in the presence of two electronegative substituents on a carbon atom of the synclinal arrangement of two bond over antiperiplanar preferred. The carbon atom to which the respective substituents are bonded is called the anomeric carbon. In the equation, the formation of molecule 2 with synclinal arrangement of red bonds compared to 1 preferred.
This stereoelectronic effect also occurs for each of the molecules shown on separately, as they can invert in her chair conformation. An axially stationary hydroxy is thus transferred to an equatorial and vice versa; also in this case is that the orientation in which the OH group is axially, preferably in both cases.
The anomeric effect was first observed in pyrans.
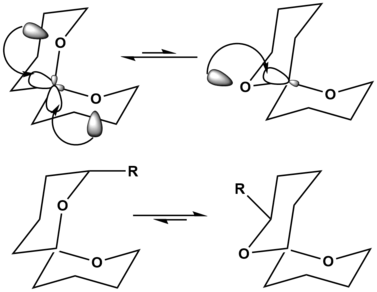
For the explanation of this effect, there are two approaches. The simpler explanation is based on classical physics. In the equatorial conformation, the dipoles of both hetero atoms are in a plane and come to the more off. The more complex explanation based on quantum mechanics and is widely recognized. Between the free electron pair of the heteroatom and the σ * orbital of the CX bond negative hyperconjugation takes place, which is energetically favorable. This is illustrated in the following diagram, wherein the atomic numbers of the oxygen atoms and the second lone pair of the rear oxygen atom are hidden for clarity.