Arrhenius plot
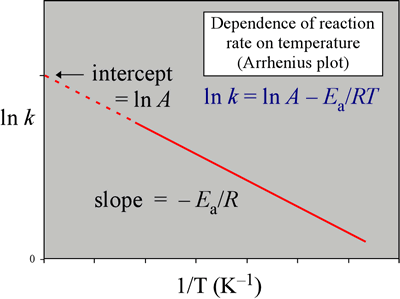
As Arrheniusgraphen (also Arrheniusdarstellung or Arrhenius plot ) refers to a graphical representation, in which the values of a quantity (such as the rate constant of a chemical reaction) logarithmically plotted against the reciprocal of the temperature. This exponentially dependent on the reciprocal temperature values are mapped to a straight line. One speaks in this context generally also of the linearization of a function.
Application
This type of application is used in chemical or physical processes, the mathematical description is the Boltzmann constant in the form of
Or
Contains as a term.
In this representation, the dependence of the measured quantity on the temperature is displayed as a straight line whose slope is inversely proportional to the activation energy EA. Based on the linear equation
The slope of the expression and the reciprocal of the temperature corresponds to the x value.
Examples
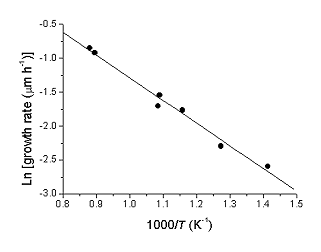
Laws of nature, which are represented in the linear Arrhenius plot, the temperature dependence of the reaction rate ( Arrhenius equation), the diffusion coefficient in solids as well as the charge carrier density in semiconductors. Taking the natural logarithm of the Arrhenius equation:
The activation energy by
Be calculated from the slope of the line.
Although the basis of experimental observations, a corresponding law is presumed this assumption can be assessed with the help of Arrheniusdarstellung.
Swell
- Physical Chemistry # literature
- Kinetics (chemistry)