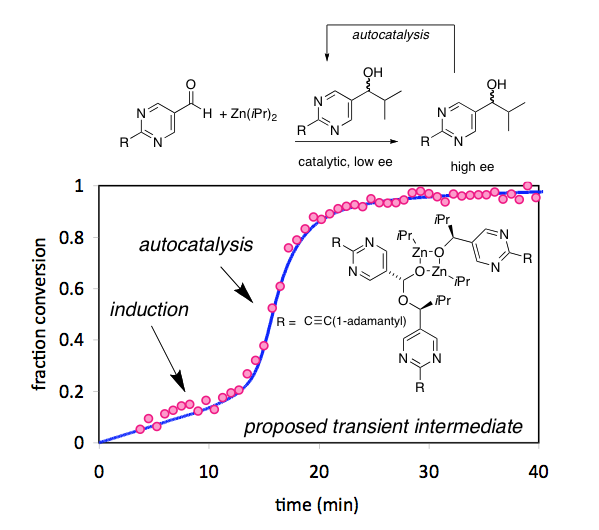
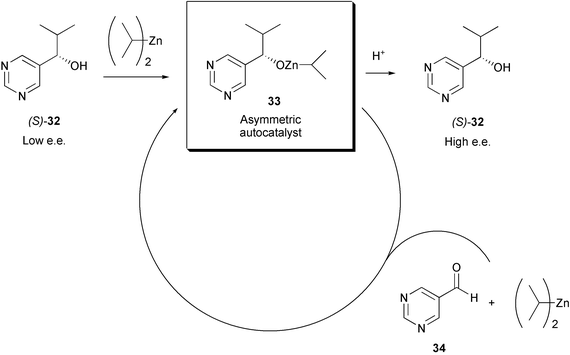
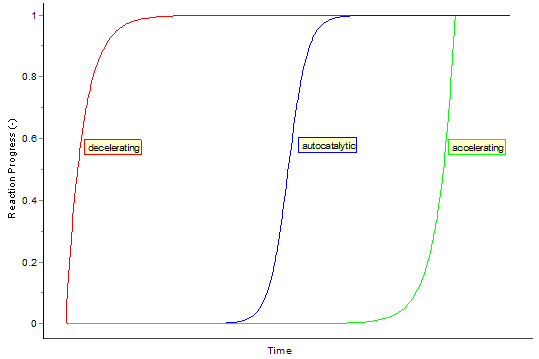
Autocatalysis
Autocatalysis ( gr αυτοκατάλυση, aftokatálissi: " self-dissolution " ) refers to a particular form of catalytic chemical reaction in which an end product as a catalyst for the reaction acts. By the continuous formation of this catalyst, the reaction is constantly accelerating. This is a positive feedback.
Example
Redox reaction of oxalic acid and permanganate:
The resulting manganese ( II) ions act as a catalyst for this reaction, so that the discoloration of the initially reluctant permanganate runs faster. Are provided for the start of the reaction of manganese (II ) ions available, so the reaction is already fast at the beginning.
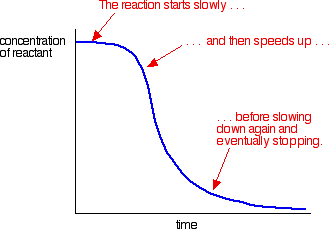
Time Act autocatalytic reactions
Car catalysis are second-order reactions:
Is.
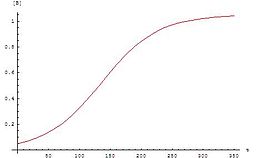
The concentrations of A and B change as follows:
And
.
The graph of this equation follows the typical for autocatalytic reactions sigmoid function: The chemical reaction starts slowly, because initially only a small concentration of catalyst is present. The rate of reaction increases steadily as with the reaction progresses, the concentration of the catalyst. Then the velocity decreases again, because the concentrations of the starting materials decrease.