Bamford–Stevens reaction
The Bamford - Stevens reaction - even Bamford -Stevens - Shapiro olefination - is a response from the field of organic chemistry. It is used for synthesis of olefins from ketones with the same number of carbon atoms in the starting material and product. The names of reaction is named after their discoverers, William Randall Bamford and Thomas Stevens Stevens ..
Survey
The Bamford - Stevens reaction allows the conversion of a ketone to an isomeric mixture of cis -and trans- alkenes:
The Bamford - Stevens reaction is carried out differently: as the base or sodium alcoholates are used as in the Shapiro reaction lithium alkyls. Depending on the base in a protic or aprotic solvent. In protic solvents, mixtures of (Z ) / ( E) - isomer, whereas the formation of (Z)- alkenes in the aprotic solvent preferably.
Reaction mechanism
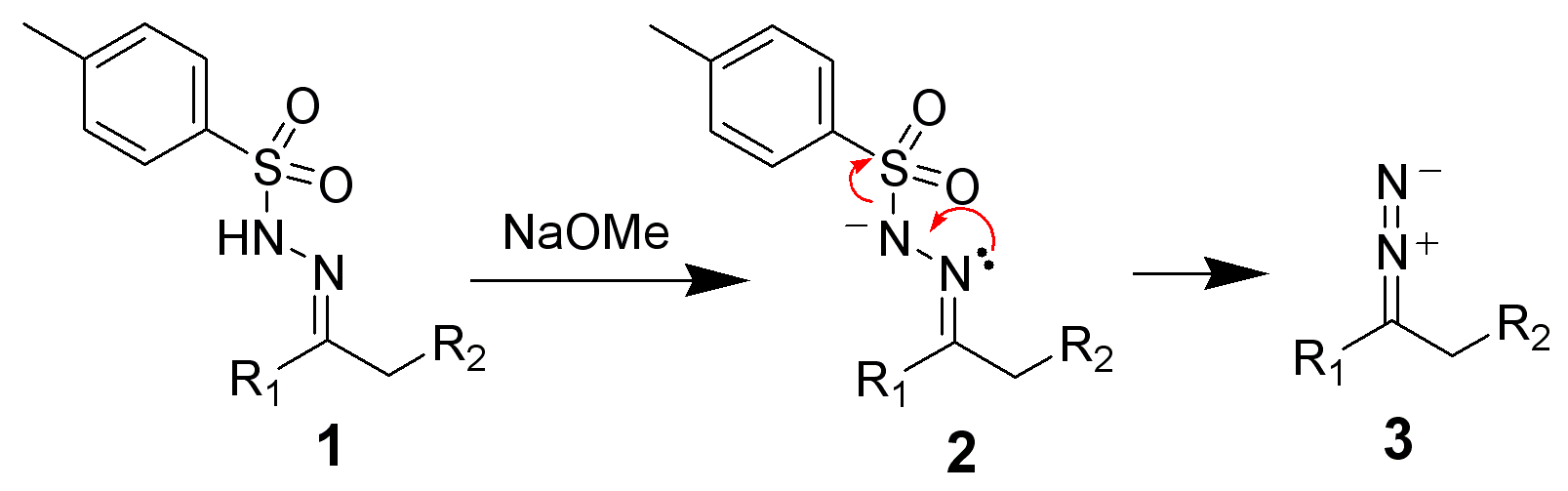
The following representations, based on the literature, a plausible reaction course. The mechanism is exemplified with butanone and methoxide as the base. Ar denotes an aryl group, for example a phenyl group. First, the butanone ( 1) is reacted with a sulfonyl Arylsulfonhydrazid to 2. This is deprotonated by the methoxide:
With elimination of the Arylsulfinats the diazo compound is 3 for the remainder of the reaction is crucial, whether you work in a protic or aprotic solvent.
Further reaction in protic solvents
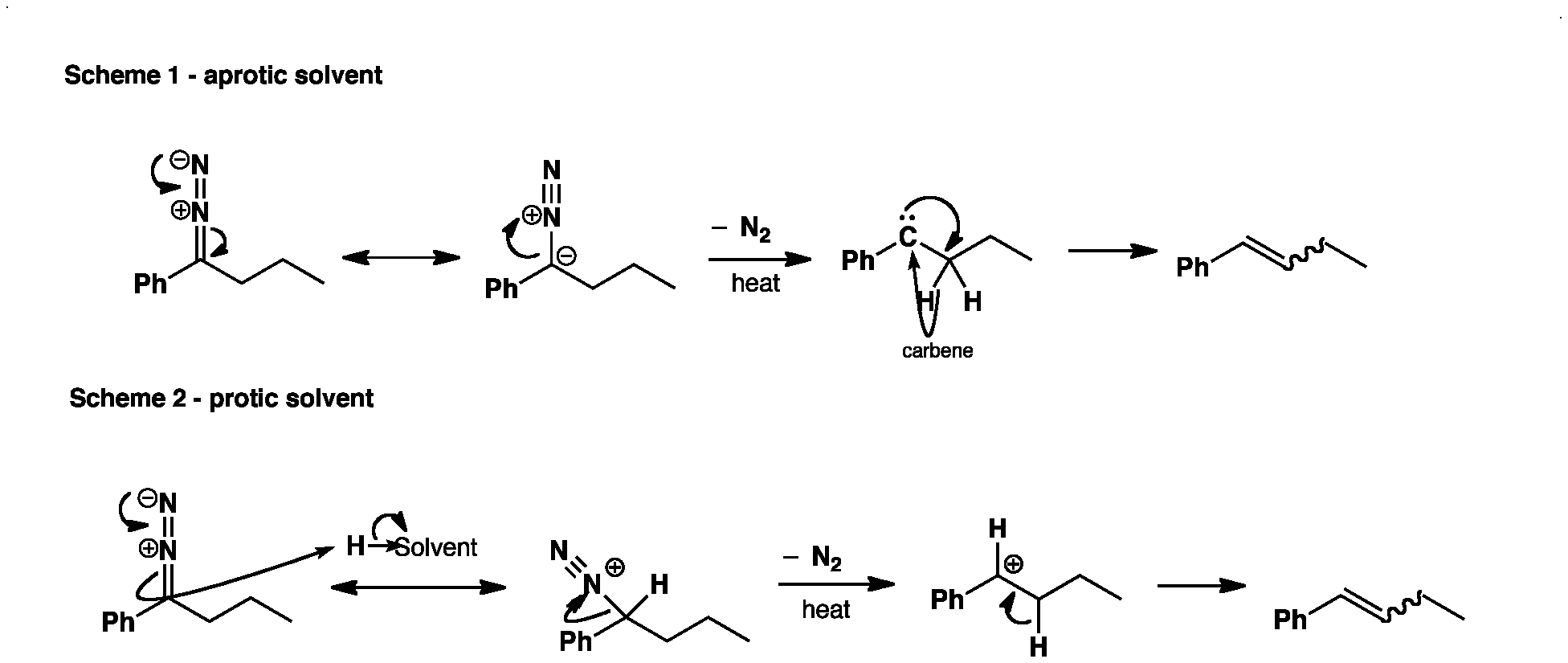
In protic solvents, a proton is abstracted in the next step from the solvent molecule. Here, the diazonium salt formed 4:
With elimination of molecular nitrogen, the carbocation 5 is formed from it. Deprotonation then leads to the alkene. The elimination of the double bond is non-selective, depending on the stability of the carbocation. It formed mixtures of (E) - isomer 6a and (Z)- isomer 6b.
Further reaction in aprotic solvents
The reaction is carried out in aprotic solvents such as formed from the diazo compound 3, with elimination of molecular nitrogen carbene 7:
7 reacts with a hydride shift preferably to the (Z)- alkene 6b.
Practical significance
The Bamford-Stevens reaction is a pure laboratory procedures. Due to the formation of stoichiometric amounts of several waste materials the atom economy of the Bamford-Stevens reaction is so bad that no one realized an industrial synthesis of alkenes based on this reaction.