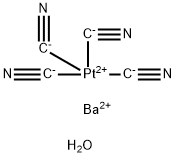
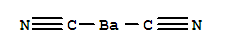Barium cyanide
Deliquescent prismatic crystals
Fixed
Good in water ( 800 g · l-1 at 14 ° C )
Risk
Template: Infobox chemical / molecular formula search available
Barium cyanide is the barium salt of hydrocyanic acid.
Production
Barium cyanide can be prepared by passing hydrogen cyanide in aqueous barium hydroxide.
It can also be prepared by annealing of barium hydroxide and carbon in the air or in a nitrogen stream.
Properties
Barium cyanide is a hygroscopic compound and crystallizes as the dihydrate Ba ( CN) 2 · 2 H2O in the form of prismatic crystals that release their water of crystallization at 100 ° C. When heating Ba ( CN) 2 in water vapor flow produced barium hydroxide Ba ( OH) 2 under expulsion of ammonia NH3 and CO carbon monoxide.
By heating with magnesium powder under airtight Bariumcarbid arises.