Barton–McCombie deoxygenation
Barton -McCombie deoxygenation ( shortened Barton deoxygenation ) is a name of reaction in organic chemistry. With the help of organic residues can be removed, for example carboxylic acid chlorides. The reaction is named after Derek HR Barton and Stuart W. McCombie.
Survey
The reaction allows the synthesis of an alkane from a carboxylic acid chloride.
Mechanism
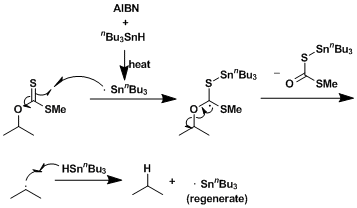
The mechanism proceeds in two steps. First coupled to a carboxylic acid chloride with an thiopyridine. The resulting product is with a radical initiator in the second step - such as AIBN or peroxide - are added.
First, a carboxylic acid chloride is added to 2- 1 Thioxopyridin -1 ( 2H)- olate 2. The carboxylic acid chloride is attacked by the rearrangement of an electron pair of the nitrogen of the 2- Thioxopyridin -1 ( 2H)- olate 2, whereby the molecule 3 forms. The alcoholate as newly formed outsourced to a pair of electrons. Thus, the chloride is firstly removed from the molecule and on the other, a carbonyl group of 4 This carbonyl group is now attacked intramolecularly of the alcoholate of the pyridine ring to give a five-membered ring in the molecule forms 5 through a further pair of electrons surroundings eventually forms the desired intermediate molecule 6 containing an ester group.
First, a radical initiator is to tributyltin hydride 7 was added. The mechanism is described here as an example with a isobutyronitrile radical, which is produced during the decomposition of AIBN. Characterized a tributylstannane radical ( 8), and the protonated residue of the radical initiator is formed. The radical 8 is then added to the previously formed pyridine derivative 6. This produces, on the one 2 - [( tributylstannyl ) thio ] pyridine and a carboxylic acid radical 9 by shifting electrons into the carboxylic acid radical decomposes it. It arises, first carbon dioxide and a radically safely rest 10 The remaining 10 radical then reacts again with a tributyltin 7 so that you get the rest in protonated form 11, then on again tributylstannane radical. The product 11 is thus a molecule in which a carboxylic acid chloride group 1 was replaced by a hydrogen atom.
Atom economy
Although the Barton - McCombie deoxygenation yields the desired product, but must also be taken into account that in this reaction considerable stoichiometric quantities of waste arising. Thus, the reaction is effective, but has a poor atom economy. So the remnants of the radical initiator must ( here: isobutyronitrile ), and the thiopyridine be disposed of. This makes the Barton deoxygenation uneconomical as an industrial process, but it can be used for the synthesis in the laboratory.