Bergman cyclization
The Bergman cyclization ( technical terminology ) and Bergman cyclization is a response from the field of organic chemistry. It is a thermal rearrangement with short-lived radical intermediates. It was named after its discoverer, the American chemist Robert George Bergman, who published it in 1972. The reaction is the synthesis of benzene derivatives from conjugated enediynes in the presence of hydrogen donors.
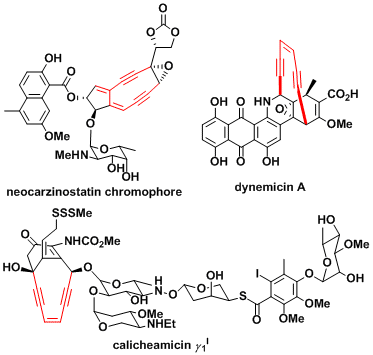
The Bergman cyclization explains among other things the mechanism of action of enediyne antibiotics. Where the radical intermediate in the inserted DNA.
Mechanism
As the hydrogen is practically often 1,4- cyclohexadiene used (as in the example above), which is implemented by emitting two hydrogen radicals to benzene. This does not interfere with a further in the reaction. The more triple bonds apart spatially, the higher the required temperature; with terminal alkynes, the temperature may exceed 200 ° C. Alkynes, who already have a cyclic Präorientierung, can usually be made to react at much lower temperatures. To achieve cyclization at physiological temperatures, should the enediyne unit part of a ten ring be (n = 1 in the example below).