Bismuth pentafluoride
Bismutpentafluorid
White solid
Fixed
5.40 g · cm -3
151 ° C
230 ° C.
550 ° C
Reacts with water
Risk
Template: Infobox chemical / molecular formula search available
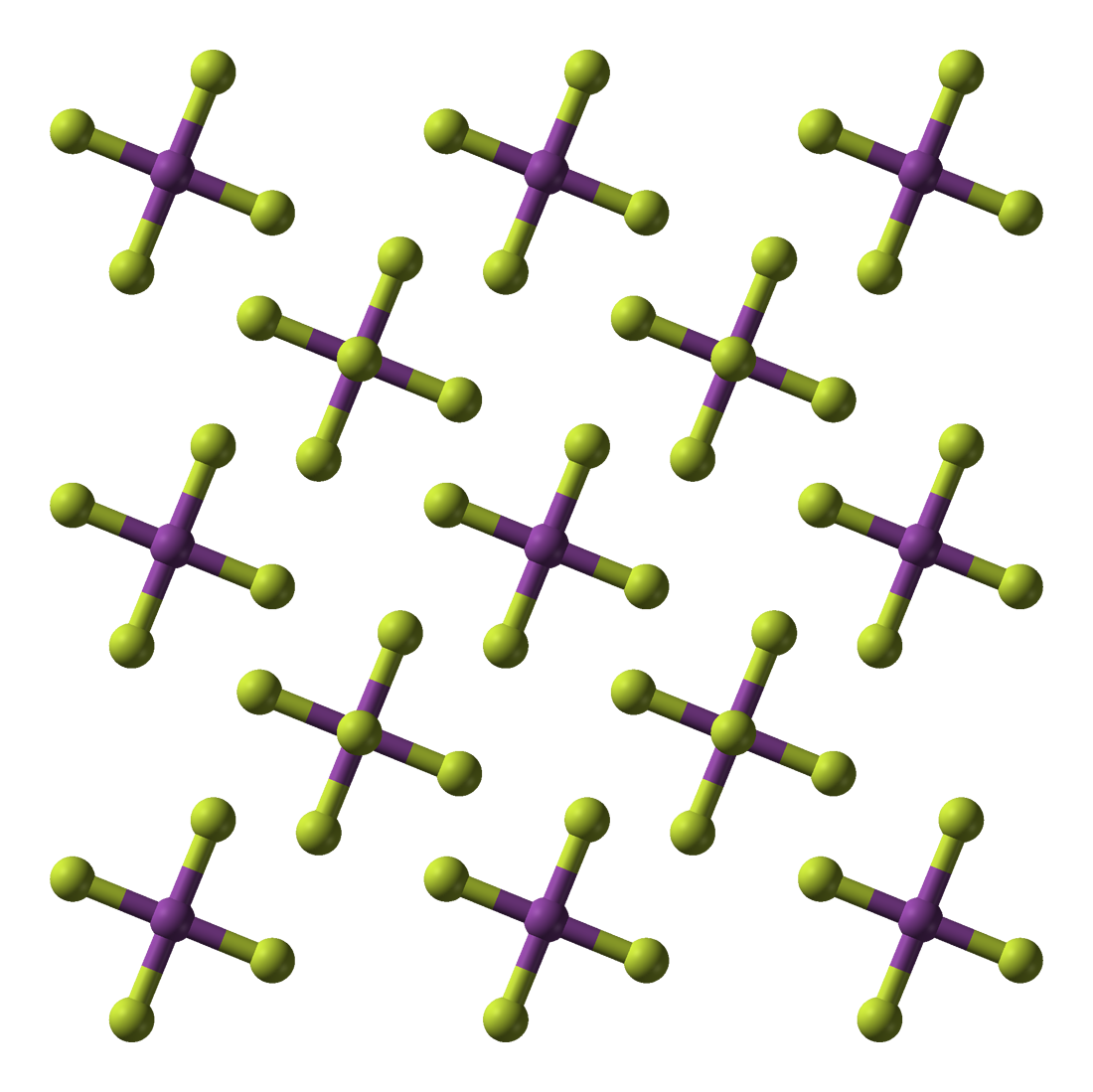
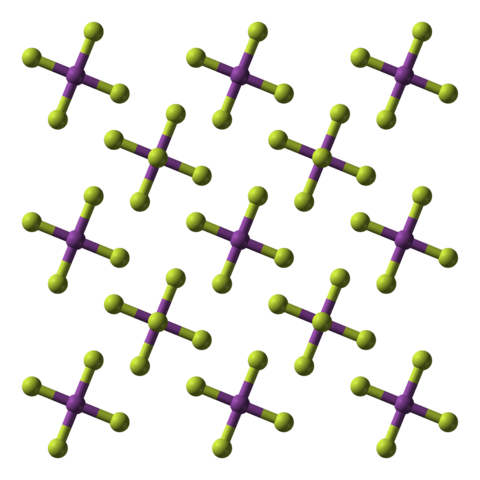
Bismuth ( V ) fluoride is an inorganic chemical compound of the bismuth from the group of fluorides.
Production and representation
Bismuth ( V ) fluoride can be obtained by reaction of bismuth (III ) fluoride and bismuth fluoride at temperatures above 500 ° C and 600 ° C.
Properties
Bismuth ( V ) fluoride is a white, highly moisture-sensitive crystalline solid. He immediately turns yellow to brown on exposure to moist air. With water it reacts sometimes under fire phenomenon, forming ozone and bismuth ( III ) fluoride. Above 50 ° C it reacts with paraffin oil. It has a tetragonal crystal structure with space group I4 / m and forming a structure with linear chains. It is an extremely strong fluorinating agent and forms with alkali metal fluorides Hexafluorobismutate (V ) M [ BiF6 ].
Use
Bismuth ( V ) fluoride as a fluorinating agent is used.