Boron tribromide
Tribromoborane
Colorless liquid with a pungent odor
Liquid
2.64 g · cm -3
-46 ° C
91 ° C
72 hPa ( 20 ° C)
Violent decomposition in water
1.312 ( 16 ° C)
Risk
-239.7 KJ / mol
Template: Infobox chemical / molecular formula search is not possible
Boron tribromide is a chemical compound consisting of the elements boron and bromine with the empirical formula BBr3.
Representation
Boron tribromide can be represented: 1 by the reaction of boron trifluoride with aluminum bromide
2 by the reaction of boron carbide with elemental bromine in the quartz tube at elevated temperature
Properties
Boron tribromide is a very toxic fumes in moist air connection, which is present at room temperature as a liquid. It is commercially available and a strong Lewis acid.
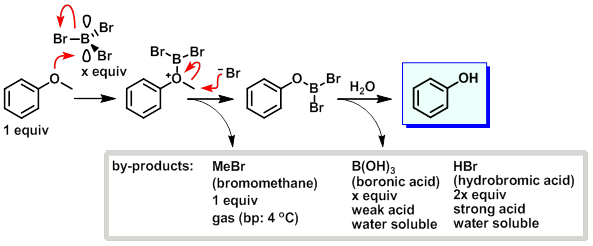
By hydrolysis is a decomposition into boric acid and hydrobromic acid:
Use
Boron tribromide can be used for the cleavage of ethers under mild conditions, in particular for mild cleavage of alkyl aryl ethers. In addition, it is also used for the olefin polymerization using Friedel -Crafts reactions. In the electronics industry, it is used as Borlieferant in doping of semiconductors.