Bouveault–Blanc reduction
The Bouveault -Blanc reaction or Bouveault -Blanc reduction is a reaction in the field of organic chemistry. It was in 1903 by the French chemists Louis Bouveault ( 1864-1909 ) and Gustave discovered Louis Blanc ( 1872-1927 ) and named after them. It is in this reaction is a reduction of esters to alcohols.
Overview reaction
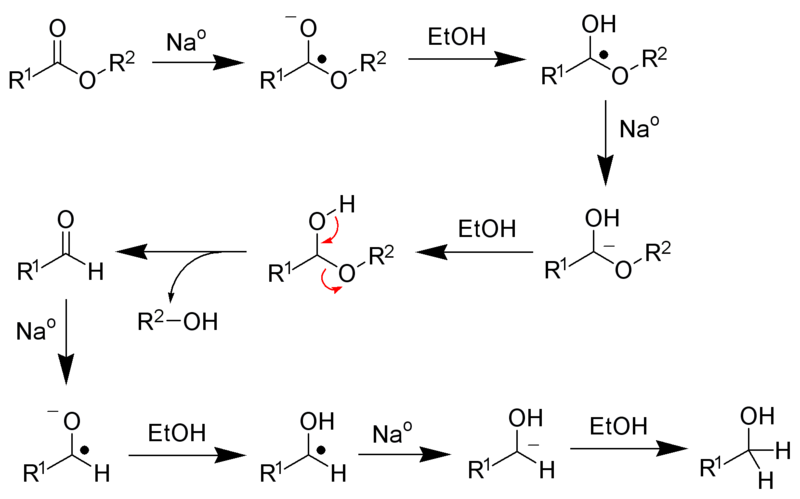
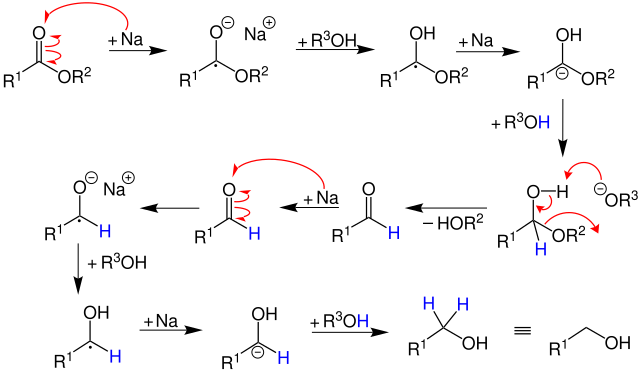
It is the reduction of a carboxylic acid ester ( R1COOR2 ) to the corresponding primary alcohol ( R 1 OH ) with the aid of metallic sodium with an alcohol - e.g. ethanol - was:
The reaction is a low cost alternative for reducing esters to primary alcohols, and is performed in industry for reasons of cost reduction, instead of using the expensive lithium aluminum hydride.
Reaction mechanism
Metallic sodium is a reducing agent with one valence electron, that is, it can only donate an electron. Thus, four sodium atoms required to completely convert an ester to the alcohol.
This reaction proceeds by a radical. By continuous reaction of the metallic sodium and ethanol is produced by a plurality of reaction steps, a primary alcohol. As a proton donor acts ethanol. Other alcohols, however, are also suitable as a proton donor.