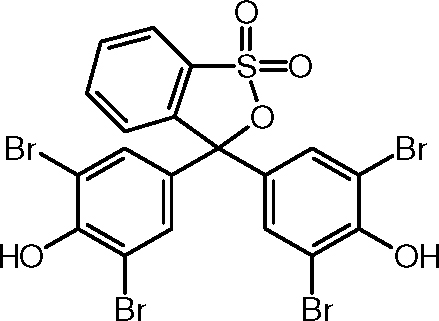
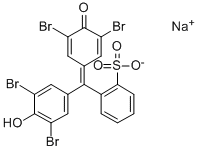
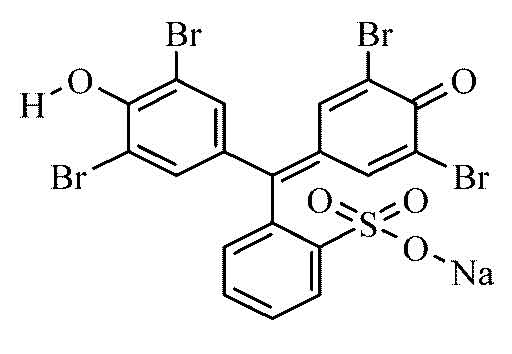
Bromophenol blue
3,3 ', 5,5'- tetrabromo- phenolsulfonphthalein
Light brown solid with phenolartigem odor
- 669.96 g · mol -1 ( acid)
- 691.94 g.mol -1 ( Na salt)
Fixed
273 ° C ( decomposition)
Practically insoluble in water
Template: Infobox chemical / molecular formula search available
Bromophenol blue is a triphenylmethane dye and is a member of the sulfonphthaleins. It is the Tetrabromderivat of phenol red. Bromophenol blue, and its sodium salt are used as pH indicators.
Properties
Bromophenol blue lashes out at a pH value of 3.0 to 4.6 from greenish yellow to blue violet. In the photometer itself shows an absorption maximum at 590-595 nm ( pH 4.6 ).
Use
- As a pH indicator: pH 3.0 (greenish yellow), pH 3.4 (green), pH 4.6 ( blue-violet ).
- As a marker dye in the sample buffer in the gel electrophoresis of DNA and proteins.
- First as invisible ink powder with which you can prepare bills or cash boxes - of any unauthorized contact created on the fingers of the person concerned an intense coloration, which is enhanced by washing.
- Is absorbed by dead sperm, of living not - important for investigations of the ejaculate.
- In retinal surgery for staining of naturally existing or morbidly gliosis resulting membranes to remove these targeted
- In cataract surgery with the color of the capsular bag
- In corneal surgery for the differentiation of individual tissue layers in the transparent cornea