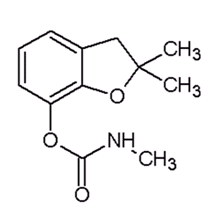
Carbofuran
- 2,3-dihydro- 2 ,2-dimethyl -benzofuran- 7-yl - methylcarbamate (IUPAC)
- Furadan
- Curaterr
Fixed
1.18 g · cm -3
153-154 ° C
Decomposition from 295 ° C
Very poor in water ( 320 mg · l-1 at 25 ° C)
Risk
- 5 mg · kg -1 ( LD50, rat, oral)
- 885 mg · kg -1 ( LD50, Rabbit, transdermal)
Template: Infobox chemical / molecular formula search available
Carbofuran is an insecticide, acaricide and nematicide with a broad spectrum of activity. It belongs to the group of carbamates.
Synthesis
Carbofuran is produced in a multistage reaction of catechol, 3-chloro -2-methylpropene and methyl isocyanate.
Effect
Carbofuran is a systemic insecticide for effective as stomach and contact poison. Its action is based on inhibition of cholinesterases acetylcholinesterase ( AChE) and butyrylcholinesterase ( BuChE ).
Use
Plant protection
Carbofuran is no longer permitted under a decision in June 2007 for the area of the European Union as an active ingredient in pesticides.
In Austria it could be used in commercial horticulture and for pickling beet seeds. Since December 2008, the sale, use and possession in Austria are prohibited. In the Federal Republic of Germany carbofuran was not an approved active substance, but an exemption has been granted, if necessary. Carbofuran was approved in Switzerland against a variety of insect pests in commercial horticulture and the cultivation of corn, beets, mushrooms and onions. At present (2013 ) no preparation is approved with this agent in Switzerland.
Toxicity
As LD50 were 8.2 to 14.1 mg in the rat · kg -1 body weight, determined · kg -1 body weight in dogs 19 mg. In long-term studies (rat, 2 years) the NOEL was 10 mg · kg -1 feed, from 100 mg · kg -1 feed stepped on a growth depression.
Environmental behavior
The half-life for degradation in the soil is from 8 to 13 days. The mobility in soil is high. Carbofuran is bee- toxic.