Carbonate–silicate cycle
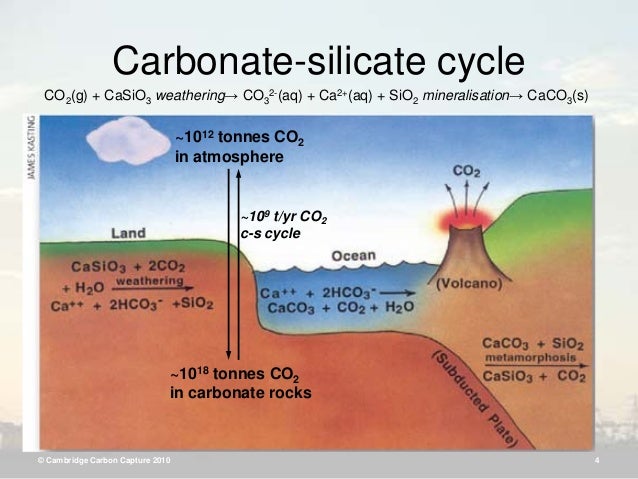
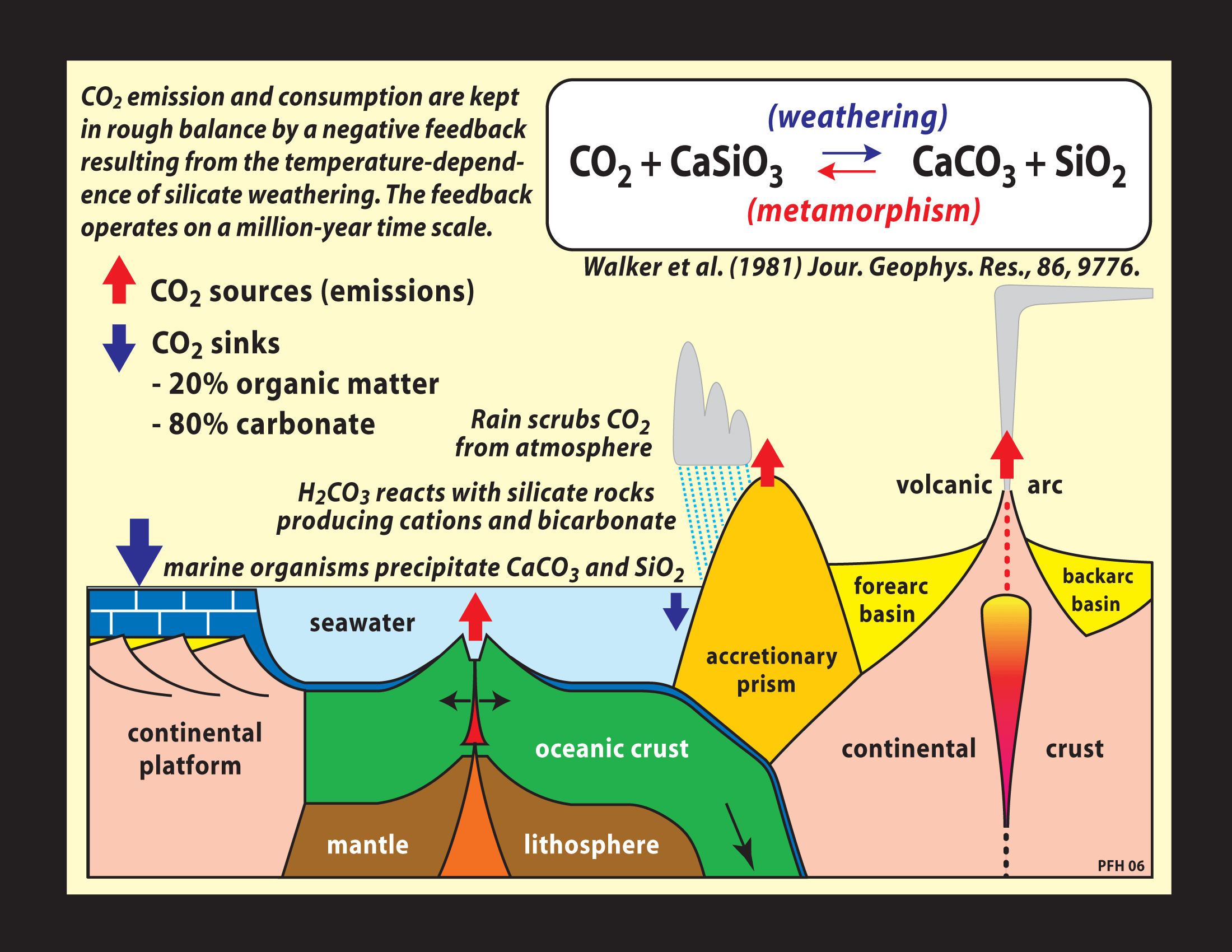
With carbonate - silicate cycle is known in chemistry a geochemical, cyclic alternation of silicates to carbonates ( and vice versa) under the influence of carbonic acid and silica. The carbonate - silicate cycle produces about 80 percent of the carbon dioxide that is exchanged between the rock and the atmosphere in the course of an estimated more than 500,000 years.
This cycle is just as important for the petrology as for Geoecology.
The cycle
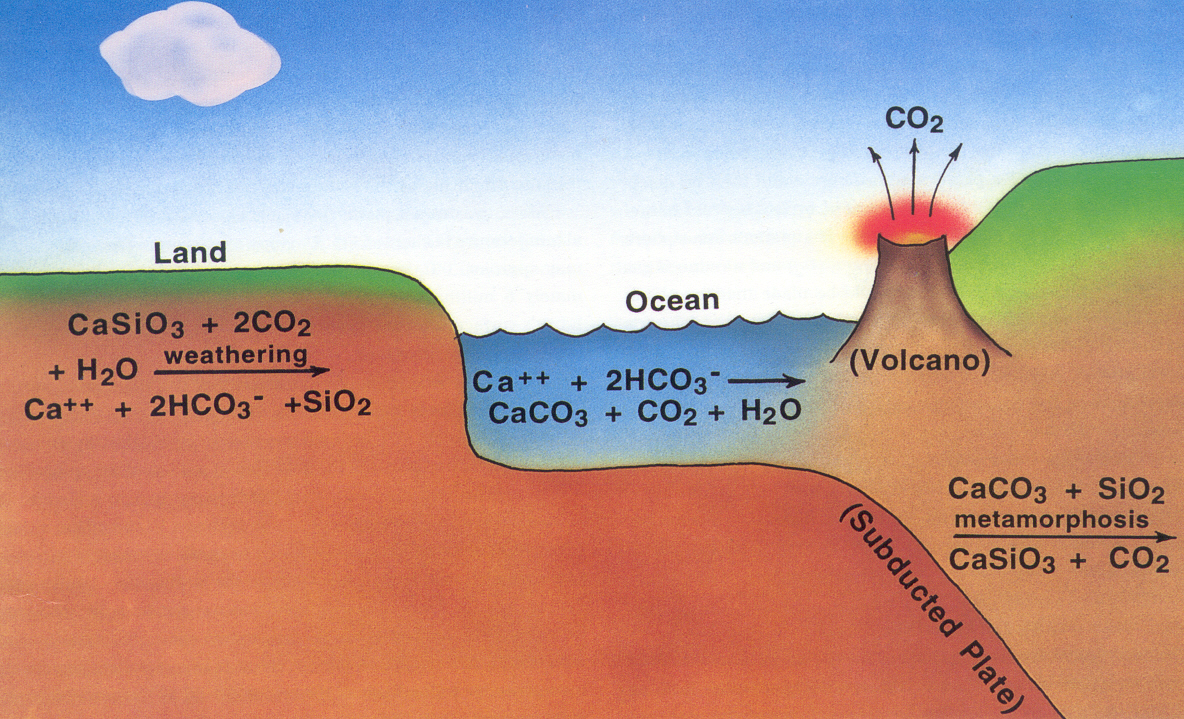
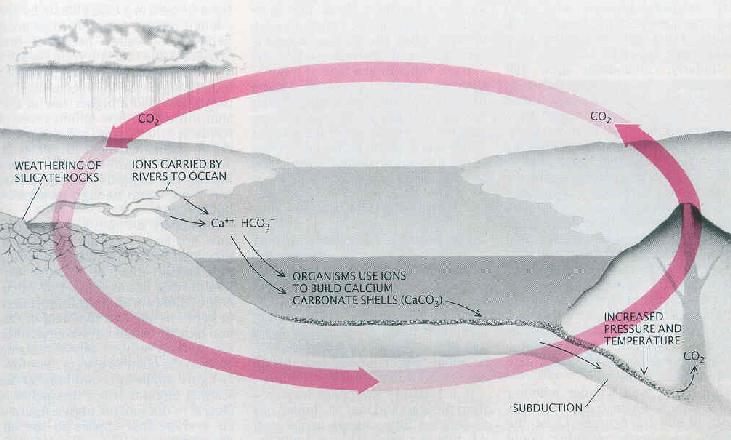
The cycle begins with atmospheric carbon dioxide and rainwater, which together form carbonic acid:
The carbonated rainwater eroded silicate rocks by it from calcium -silicate minerals ( compounds of calcium, silicon and oxygen) dissolves, thus the released calcium and bicarbonate ions enter the groundwater. As an example, the equation of the implementation of the feldspar anorthite used by carbonic acid with the formation of kaolinite:
The ions are transported by rivers into the sea. In the sea use different creatures, planktonic and sessile animals, the calcium, bicarbonate and carbonate ions to build up internal or external skeletons of calcium carbonate (CaCO3). After her death form on the seafloor carbonate sediments. Over the millennia the seabed as a result of plate tectonics of mid-ocean ridges is transported to the subduction zones at continental margins. He wanders into the earth. Due to high pressure and high temperatures, the calcium carbonate reacts with silica ( Carbonatmetamorphismus ) and are formed again under the elimination of carbon dioxide silicate minerals. As an example, the equation of the reaction of calcite (calcium carbonate) with silica to wollastonite and carbon dioxide:
The carbon dioxide finally arrives, either via the submarine volcanic activity of the mid-ocean ridges or continental volcanism, again as carbon dioxide into the atmosphere, thus closing the cycle is closed.