Carrier generation and recombination
Recombination in plasma physics, the neutralizing association electrically positive and negative charge carriers (ions, electrons). Recombination represents the reverse process to Ionization
The ionization requires energy supply, which is mainly due to high temperatures photons or other particles by impact ionization. Thus, encounters charged particles themselves can recombine, this energy must be released again. This can be done by Emission of Radiation ( Rekombinationsleuchten, eg, lightning, meteor, LED), by energy transfer to other particles by dissociation into neutral particles or in the form of lattice vibrations ( acoustic phonons).
Recombining in the gas phase
An ionized gas is a plasma. Examples are gas discharges and the ionosphere.
If there is enough pressure and the triple collision ionization - recombination is dominant, in which a positive ion with two electrons pushes the same time. The ion recombines with the first electron to a neutral atom. The released binding energy is " dissipated " by the second electron ( similar to the heat radiation for inelastic collision ). The second electron is increased in the process of its energy. Alternatively, another atom or molecule include the binding energy. Due to the lower range of the interaction with neutral particles, this process is competitive only at a low degree of ionization.
Is the pressure of the neutral gas low, so a wall or particle surface as a catalyst convey ( Wandrekombination ). She takes on not only the binding energy but also increases the probability of an encounter, if electrons or ions on the surface dwell ( adsorbate ). The Wandrekombination is particularly effective in electrically conductive wall.
In the ionosphere, the pressure is low, and no wall in the vicinity. There outweigh two types of recombination:
You can only take place where either molecular ions arising directly or through the ion-atom exchange which requires neutral molecules.
However, is a very slow process and can be ignored in the ionosphere on accounting. Richer forms of energy can be considered as a transition to secondary radiations. Aurora is no recombination, based on collisional excitation, collisional ionization is not.
Recombination in semiconductors
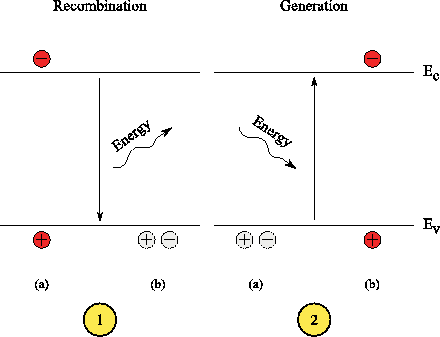
In the semiconductor is called recombination, when an excited electron into the conduction band relaxes again, that is, with the release of a photon or phonon ( lattice vibration ) into the valence band " falls back ". One speaks of radiant or nonradiative recombination. In a light-emitting portion of the charge carriers recombine radiant. In silicon semiconductor devices ( indirect band gap) on the other hand takes place almost exclusively non-radiative recombination.
The opposite process of recombination is the generation, during the ionization by means of an electron and a hole is generated. The ionization energy mostly originates from photons or phonons. The recombination and generation rates will always be assumed in thermodynamic equilibrium as equal.
In the recombination are often selected a simple approach for the recombination, i.e., the number of recombinants per unit of time ( and volume).
For electrons applies:
Analogy applies to defect electrons:
N and p in this case denote the concentrations of the charge carriers (electrons or electron holes ), and the equilibrium concentrations and or the effective lifetimes of the carriers. Thus, clearly increases the recombination rate when the carrier concentration is higher than the equilibrium concentration.
More specifically, there are many different effects that play a role in the process of recombination.
Photons or phonons, whose energy (E = h · ν ) is greater than the energy gap Eg in the semiconductor can give their energy to valence electrons, thus generating electron -hole pairs in the semiconductor. These charge carriers ( electrons and holes) pass through radiation and / or lattice vibrations ( phonons) again in the direction of the band edge, since there their energy is minimized. This effect limits significantly the efficiency of solar cells, but it can be reduced by the use of a tandem solar cell.
Recombination of electrons and holes can be either radiative or non-radiative. Recombining them radiant, so you call this effect luminescence. It is crucial that for an observable radiative recombination a direct semiconductor is necessary where there is no difference in the pulse - Bandminima (see band model ).
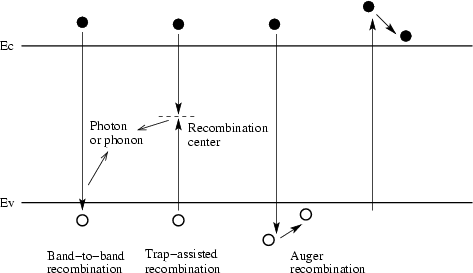
There are three known Rekombinationsarten: Radiant, Shockley - Read-Hall and Auger recombination.
Radiant recombination
Here recombine an electron beam with a hole. The resulting photon having the energy E = h · ν, which is at least as great as the energy of the bandgap. As a formula for the radiative recombination, the charge carrier density is set at a material-dependent constant Rekombinationsfaktor Cdir mostly. It reads thus, with the carrier densities n, p and the intrinsic carrier density ni:
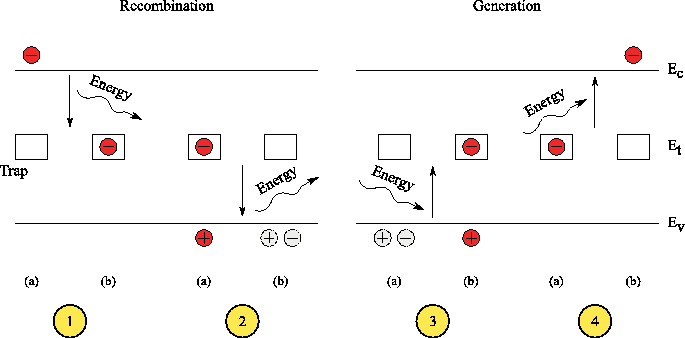
Shockley - Read-Hall recombination
In this recombination, the electron first jumps on a Rekombinationsniveau, which is located approximately in the middle of the band gap, and recombine it with another jump with a hole under liberation of thermal energy in the form of lattice vibrations. The energy levels in the band gap caused by defects in the crystal lattice, such as dopants. Since recombination via a belt level requires less energy, it is usually more likely than the direct recombination. Defect atoms are thus recombination centers or traps or traps ( trap german ) for free carriers. In the SRH recombination so it is a non-radiative recombination. You can be the charge carrier lifetimes and start:
The sizes nd and pd are defined as follows ( Rekombinationsstörstelleenergie ET, Fermi energy EF, temperature T, Boltzmann constant kB):
Auger recombination
Auger recombination is also a non-radiative recombination. A conduction band electron are indeed its energy by jumping into a hole in the valence band off, but this energy is completely absorbed by another conduction band electron. This electron relaxes back to either the conduction band minimum, there is again in the form of its energy from lattice vibrations so as to minimize its energy, or leaves in the vicinity of the crystal (see Auger electron spectroscopy).
Recombination on the surface
This is a recombination by unbound (also: unsaturated ) states at the surface of the semiconductor. This unbound states (English: dangling bonds ) on the one hand cause additional states in the band gap, can recombine on the electrons and holes. On the other hand, because impurities (dirt, moisture, etc.) can be set. The recombination process via unbound states is harmful to devices generally and reduces their lifespan. In addition, the conditions at the surface is no longer well-defined, reducing the predictability of a semiconductor device. However, this effect wins by its targeted technical utilization is becoming increasingly important.
For the calculation can be an approximation of the SRH recombination use, which does not have a discrete trap site ( trap ) is recombined here, but on a whole spectrum of recombination in the resulting forbidden zone at the surface.
The surface recombination rate calculated to be:
: Cross section for recombination
: Surface state density at the center of the forbidden zone
As a technical surface passivation method to saturate the unbound surface states are called. In silicon, this is usually achieved by a thermally grown silicon dioxide. Passivation can also be achieved by means of silicon nitride over PECVD process. Passivation layers are particularly suitable for highly efficient solar cells of interest ( rear-side ).
Unsaturated bonds continue to occur at grain boundaries of polycrystalline semiconductors. Saturation can be done for example through hydrogen, which is allowed to diffuse into the material at high temperatures with silicon.
Sources and footnotes
- Atomic physics
- Solid State Physics