Chemical energy
Chemical energy as the energy form is referred to, which is stored in the form of a chemical compound in a carrier and can be released energy in chemical reactions. The term goes back to Wilhelm Ostwald, of him in 1893 in his textbook " Chemical Energy " (in the phrase pair " Chemical and internal energy " ) in addition to other forms of energy ( " Mechanical Energy", " heat ", " Electrical and magnetic energy " and " radiant energy " ) presented.
Chemical energy is a macroscopic expression for the description of the energy that is connected to electric forces in atoms and molecules and is expressed in chemical reactions. It can be divided into kinetic energy of the electrons and potential energy of the electromagnetic interaction of electrons and atomic nuclei. It is an internal energy, such as thermal energy and nuclear energy.
Use of the term chemical energy
In technical science chemistry, the term " chemical energy " not used, only specifying the environmental conditions of the term is clearly defined - that there exists in each case a different established term.
Often with chemical energy, the energy is meant, which is released by combustion of a substance (at constant pressure ), so the heat of combustion. The set of Hess enables the calculation of the energies in transformations from the precisely defined enthalpies of formation of the compounds involved.
Similar terms are calorific value and calorific value, which aim respectively to the maximum possible at a burning heat.
Chemical energy should not be confused with the chemical binding energy. The chemical binding energy describes the strength of a particular chemical bond, thus indicates how much energy must be supplied to the molecule to dissolve the bond.
In other sciences, engineering, etc., the term of the chemical energy is widely used in its partially blurred shape. Although some physics educationalists criticize the use of the term ( " The term is useful when it comes to a rough guide, but proves to be intractable, if one tries to take him sternly. In physical jargon it is useful in the physical calculus superfluous to physical understanding hindrance. " ), the term is accepted in almost all didactic publications and textbooks and used.
Use of chemical energy in technical systems
From a technical standpoint chemical energy stored in fuels, which is converted by their combustion, eg in the propulsion of vehicles, into mechanical energy. Fuel cells allow the transformation of chemical energy of a combustion reaction directly into electrical energy. When using batteries, chemical energy is converted directly into electrical energy by electrochemical redox reactions. An accumulator behaves in the use of energy like a battery, but can also be reversed convert electrical energy into chemical and save as.
Use of chemical energy in biological systems
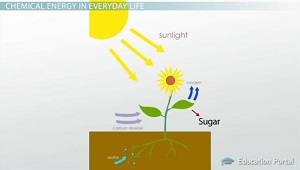
From a biological standpoint chemical energy stored in organic food, which is converted as energy in ATP molecules. Green plants do not derive their chemical energy from organic food, but from the energy content of solar radiation, some bacteria from oxidation energy reduced compounds (eg, Fe2 or CH4). The ATP molecules within biological cells allow to make chemical, osmotic and mechanical work.







.jpg)

