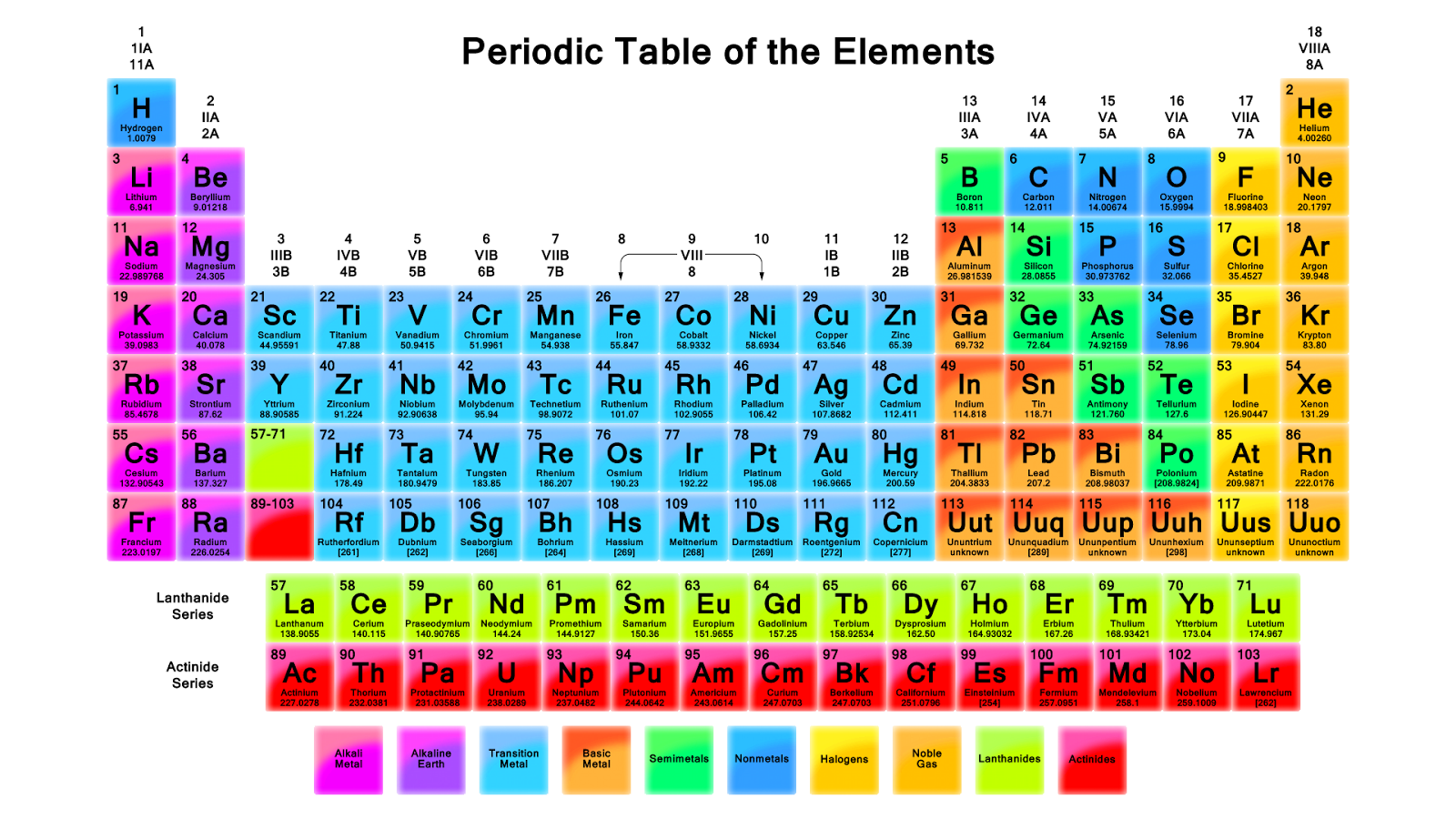
Chemical symbol
Element symbols are used as shorthand for individual atoms of chemical element and consist of one or two letters. This element symbols are internationally (see IUPAC) and are also used in countries other than the Latin alphabet use ( Greek, Cyrillic, Arabic, ... ).
The symbols are derived from the Latin or Greek names, in the recent English also by the names of the elements. If the symbol of an element of two letters, the first letter is capitalized and the other lowercase. When not yet been named elements, the name is derived from the number of the atomic number of the element, to Latin or Greek numerals are used. For example, the element with the atomic number 118 out as Ununoctium with the symbol UUO (see: systematic element name).
Element symbols in chemical formulas represent not only the type of the element, but also a certain amount of it, in each case a single atom or a single multiple thereof; usually 6.022 * 1023 atoms (see Avogadro's number ), because that is the atomic mass (formerly atomic weight ) of the element corresponding amount in grams. In contrast to single atoms are the amounts that you can weigh and handle in chemical experiments and under which each can also imagine something.
Development
More than a thousand years thought the philosophers, the earth would consist of four elements: the dry and warm fire, the warm and humid air, the damp and cold water and the cold and dry earth (Read more under the four- element theory ). Thus the circle was closed for them. To ensure that they are talking about the same item, they gave them symbols. Many centuries later, there were no discussions about this idea.
It was not until the early Middle Ages the alchemists developed mainly concerned with the production of gold and base fabrics, a kind of them to be read only secret language and secret writing. In order to record their test results, they used symbols for pure materials to create short notes. Here, every Alchemist developed his own formula language, there were few general symbols. In their works, therefore there was always a page with the same explanation of the chemical symbols.
At the beginning of the 19th century - with the development of the atomic hypothesis - John Dalton simplified this system by distinguishing first between elements and compounds. For each element was then known, he introduced a certain circle symbol, the connections he described by a concatenation of the corresponding element symbols.
1814 led Berzelius today a common symbolism. He replaced the defined symbols of Dalton by the initials of the Latin name elements. However, some of the already known elements had the same initial letter. Therefore, these items were given as a " symbol" two letters. He also simplified the spelling of Dalton in that it does not describe the number of individual atoms in the molecule, each by one single character or symbol, but the index introduced. Thus, for example, the COO now known spelling CO2.
Examples
( only the symbols notwithstanding formed by the German element name )
- Au = Aurum = Gold
- Bi = bismuth is bismuth
- C = carbon = carbon
- Ca = calcium = calcium
- Co = Cobalt = cobalt
- Cu = copper Cuprum =
- Fe = iron = Ferrum
- H = hydrogen = Hydrogenium
- Hg = mercury = Hydrargyrum
- I = iodine iodine =
- N = Nitrogenium = nitrogen
- O = oxygen = Oxygenium
- Pb = Lead = Plumbum
- Sb = antimony Stibium =
- Sn = tin = STANNUM