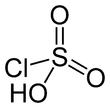
Chlorosulfuric acid
- Chlorine (O) sulfuric acid
- Sulfuric chlorohydrin
- Sulfurylhydroxylchlorid
Colorless, pungent-smelling, hygroscopic liquid
Liquid
1.75 g · cm -3 ( 20 ° C)
-80 ° C
152 ° C ( with decomposition)
0,45 hPa ( 20 ° C)
Violent decomposition in water
Risk
50 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search is not possible
Chlorosulfonic acid or chlorosulfonic acid ( HSO3Cl ) is an incomplete acid chloride of sulfuric acid, in which only one hydroxy group of the acid is replaced by chlorine. Chlorosulfonic acid is a colorless, pungent-smelling and the air strongly fuming liquid. The melting point is -80 ° C, the boiling point at 152 ° C. Chlorosulfonic acid chlorides, like all very reactive with water, it reacts violently with conversion to sulfuric acid and hydrochloric acid.
Their effect as fog agent based on the reaction with atmospheric moisture to give a mist of sulfuric acid and hydrochloric acid forms. This acidic fog is very aggressive, he attacks metals and irritating cough, as well as organic materials (wood, fabric) are attacked. If, in addition sulfur trioxide dissolved in chlorosulfonic acid, one obtains the fog acid forming denser fog as chlorosulfonic acid. The use of chlorosulfonic acid as a mist means presupposes a sufficiently high air humidity, therefore chlorosulfonic acid was used primarily in the Navy. Firstly, because of the humidity, and secondly, because the ship can be removed from the resulting fog and hence its aggressive effect is less exposed.
Synthesis
Of chlorosulfonic acid, by the action of phosphorus pentachloride on conc. Sulfuric acid are shown:
Technically chlorosulfonic illustrated by introducing hydrogen chloride gas in liquid sulfur trioxide:
Use
Chlorosulfonic acid is used in fog barrel devices to produce fog acid.
Chlorosulfonic acid is used in addition as a mist in the preparation of chemical intermediates (introduction of HSO3 - group in aromatic compounds ). Here, the aromatic compound is first reacted with an excess of chlorosulfonic acid chlorosulfonated (introduction of SO2Cl group in aromatic compounds ) and can later be hydrolyzed selectively to the sulfonic acid. The sulfonyl chlorides are not soluble in water and better suited for many reactions, in contrast to the sulfonic acids.
Further, chlorosulfonic acid used to produce ion exchange resins.