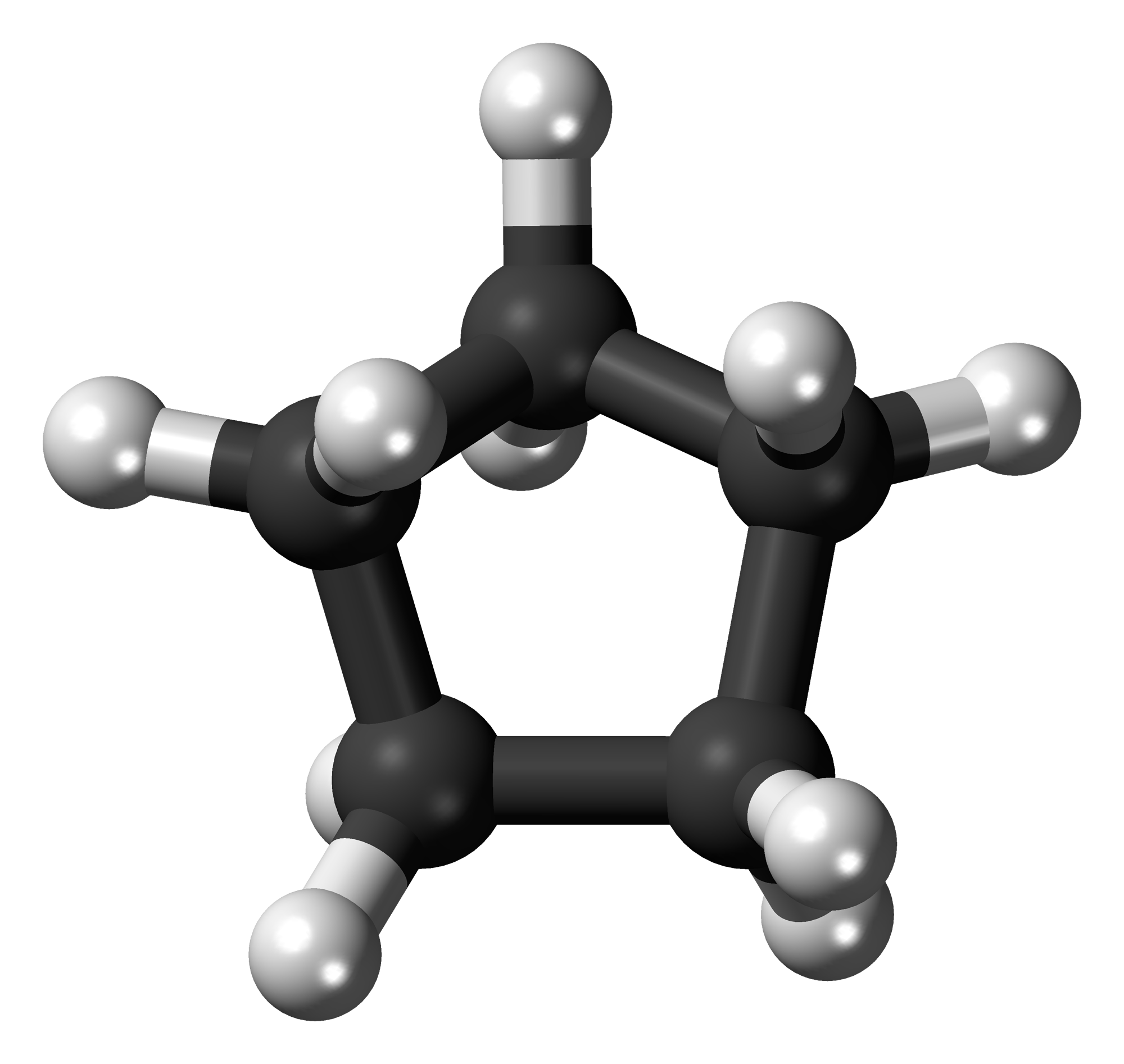
Cyclopentane
Pentamethylene
Colorless liquid with an odor benzinartigem
Liquid
0.74 g · cm -3 ( 20 ° C)
-94 ° C
49 ° C
346 hPa ( 20 ° C)
Practically insoluble in water ( 156 mg / l at 25 ° C), miscible with many organic solvents
1.4065 (20 ° C)
Risk
11400 mg · kg -1 ( LD50, rat, oral)
Template: Infobox chemical / molecular formula search available
Cyclopentane is an entity belonging to the group of cycloalkanes colorless liquid.
Properties
The skeletal formula shown in the infobox is not the "real" molecular structure. The five carbon atoms do not lie in one plane. In addition, the molecule is " flexible," that is, its atoms are in the molecule association in constant motion ( see Article pseudorotation ). The low-energy conformations are the so-called envelope conformation ( engl. envelope conformation ) and the half-chair conformation (half -chair conformation ).
Cyclopentane melts at -94 ° C and boils at 49 ° C. In water, cyclopentane is practically insoluble, but with many organic solvents, it is miscible. The vapor pressure at 20 ° C 346 hPa, the liquid is volatile. The density is 0.75 g / ml at 20 ° C. Cyclopentane smells like gasoline. The flash point is -51 ° C, the ignition temperature at 320 ° C.
Use and occurrence
Cyclopentane is found in petroleum. It is used inter alia as a solvent for organic substances. Since the 90s cyclopentane ( optionally in admixture with its isomers or other C 4-hydrocarbons ) is used as the (physical ) blowing agents in the production of rigid polyurethane foams. The majority of existing in Germany newer household refrigerators containing the hydrocarbon isobutane as refrigerant and the hydrocarbon cyclopentane as a foam blowing agent.
By the oxidation of cyclopentane to cyclopentanone can be synthesized
Safety
Cyclopentane is highly flammable and slightly hazardous for water (WGK 1). It forms as a result of its low boiling point of 49 ° C and its flash point of -51 ° C flammable vapor - air mixtures. The explosive range is between 1.4 vol% (41 g/m3) as the lower explosive limit ( LEL) and 8.0 % by volume (233 g/m3 ) and upper explosive limit (UEL ). With a minimum ignition energy of 0.24 mJ vapor - air mixtures are extremely flammable. High concentrations of cyclopentane can lead to unconsciousness.