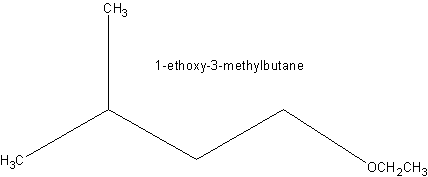Ethoxy
The ethoxy group ( ancient Äthoxylgruppe ) is one of the simplest atomic arrangements in organic chemistry, which adds an ethyl group by a heteroatom ( oxygen).
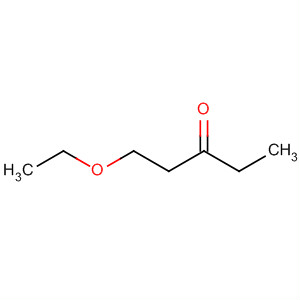
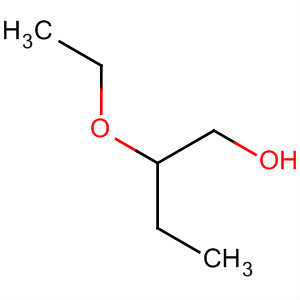
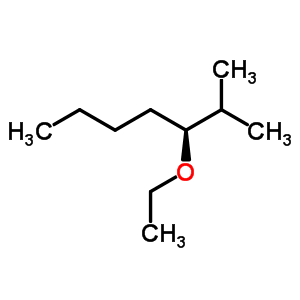
The formula of the substituent is - OC2H5, sometimes also written as -OEt.
The ethoxy group is not an independent chemical substance, but always part of a larger molecule ( see figure). The smallest molecule with this group is ethanol, which is but usually classified as alcohol with a hydroxyl group.
Properties
Is a hydrogen atom in an alkane substituted by an ethoxy group, the polarity and reactivity increases. Such ethers are cleaved with aqueous acids at elevated temperatures in alcohols. An ethoxy group has as first deputy a moderately activating and ortho (next ) or para (opposite) directing effects in electrophilic aromatic substitution.
Quantitative analytical determination
For quantitative analytical determination of a sample of the ethoxy groups on the test substance is heated by an excess of hydriodic acid at about 100 ° C, and the resulting ethyl iodide is distilled off. The latter is added to an ethanolic solution of silver nitrate and silver iodide formed due to hydrolysis of the Iodalkans determined gravimetrically.