Heat capacity ratio
The isentropic exponent (symbol: κ ) (also Adiabatenindex ) is the exponent in the equation
For the isentropic change of state of an ideal gas.
Since a change in state is isentropically when adiabatic and reversible extends, as for example in large-scale air flow is approximately the case, is called the exponent in meteorology often adiabatic, adiabatic or Adiabatenindex. The technique is usually an adiabatic change of state ( eg in a steam turbine ) is not isentropic, since friction, choke and shock processes produce entropy (see " adiabatic engine " and " Second Law of Thermodynamics" ). These state changes can be approximately described by a polytropic with a variable polytropic exponent n.
The Isentropic is the special case of a polytropic with (see picture).
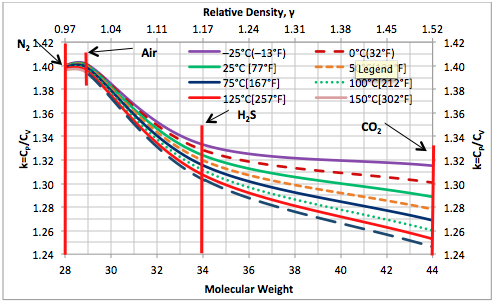
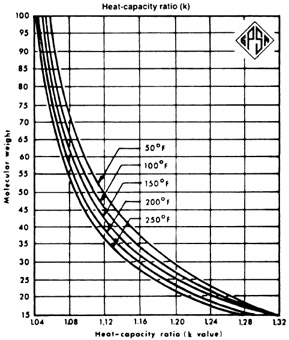
The isentropic exponent is defined as the ratio of heat capacity at constant pressure (cp) to the heat capacity at constant volume ( cV ) and corresponds to the temperature-induced gas expansion. The value depends on the degree of freedom of the gas. Gas molecules with more atoms have a higher degree of freedom. The degree of freedom consists of translation, rotation and oscillation degree of freedom. Translation is excited at all temperatures, rotation only from the middle, vibration at higher temperatures. In other words: " freeze " with decreasing temperature, an increasing number of degrees of freedom. Therefore, the heat capacity of polyatomic gases to a strong temperature dependence. Also, the isentropic exponent of polyatomic gas molecules is strongly temperature dependent, since the difference between isobaric and isochoric heat capacity for all gases over a wide temperature range remains constant.
The number of degrees of freedom of a gas molecule is dependent on the geometry and the bonding strength of atoms.
The degree of freedom of a body is the number of possible movements of the body within a coordinate system has. The single ground point has three degrees of freedom, it can move along the x-, y -and z- axis in the space. He has no rotational degrees of freedom, because a point can not rotate. A system of n points 3N has degrees of freedom. If there are between the points r rigid bonds, this reduces the number of degrees of freedom.
The isentropic exponent of dry air is under normal conditions κ = 1.402 and is thus close to 1.4, in which the theoretical value of 3 translational and 2 rotational degrees of freedom ( in diatomic molecules in this sense is any rotation about the connection axis is possible) corresponds. Vibrational states are thus hardly excited. At much higher temperatures it is in addition to the molecular vibrations of dissociation and ionisation to more degrees of freedom. In humid air can cause water loss, for example due to cooling during expansion: by the released heat of condensation of the exponent is lower.
Trade shows can be the isentropic exponent using the Rüchardt experiment.