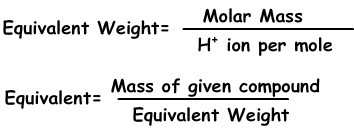Normality (chemistry)
The equivalent concentration (symbol: ceq ), outdated normality ( unit symbol: N) is an indication of concentration in chemistry. It is a specific amount of substance concentration, which is based on a reference factor 1 / z. Here, z is the stoichiometric value, which is also called equivalent number. In case z = 3 is thus the equivalent concentration three times as high as the molar concentration, as it were, each of all the particles is Z times counted. The term 1 / z is also called Äquivalentteilchen or equivalents. ceq is a measure of how many Äquivalentteilchen of a substance are in a certain volume of the solution.
Especially important is the equivalent concentration is in neutralization reactions, redox reactions, in volumetric analysis and ion reactions.
In a redox reaction, a permanganate of MnO4 -5 electrons absorb a chloride ion but only emit an electron. The molar mass of potassium permanganate must be divided by 5, then the amount to be solved in exactly one liter of distilled water to 1 neq (mol electron uptake ) to obtain the equivalent concentration of this oxidant / liter. 1 neq (= 1 eq) electron uptake corresponds to, ie 1/5 molar mass of KMnO4 and this is described as: neq ( KMnO4 ) = n (1/5 KMnO4 ).
With acid / base titrations, there are acids with one or two (e.g., sulfuric acid) or three protons (e.g., phosphoric acid). These acids titrated with sodium hydroxide solution, is needed according to the number of equivalent of the acid, one, two or three parts of sodium hydroxide solution to neutralize the acid. Therefore, in acid / base titration the molar mass divided by the number of deliverable or recordable protons and this amount of substance least one liter. Dissolved water, to obtain the equivalent concentration of 1 mol of protons neq. Just then: neq (H2SO4 ) = neq (NaOH )
Definition
The equivalent concentration is defined by the equation:
This is the equivalent concentration ceq and c is the molar concentration of the solution and z is the stoichiometric value, also equivalent number.
The stoichiometric value - and thus the equivalent concentration of a given solution - may be dependent on the chemical reaction, thus the use of the solution, without these changes themselves. Further, the equivalent concentration is temperature dependent.
Customary unit is mol / liter.
Solutions with ceq = 1 mol / L were formerly referred to as "normal solutions ". They spoke also of 0.1 N solutions, if ceq = was 0.1 mol / L, etc.
Another way is the definition of the number of solute in one liter of a solution or equivalents Val
Normality is then defined by the equation:
Here, N is the normality of the amount of equivalents, and V is the volume.
Examples
Salt solutions
Sodium carbonate ( Na2CO3) is composed of two sodium ions (Na ), and a carbonate ion. Thus corresponds to a 1 molar (M) sodium carbonate solution, a 2 N (N) solution of sodium carbonate based on the sodium ion (Z = 2).
Acid -base reactions
In acid -base reactions are Äquivalentteilchen protons (H ) in acid solutions or hydroxide ions (OH - ) in alkaline solutions. For example, two protons can attach to a sulfate ion ( SO42 - ), which corresponds to the valence of the acid anion. In the solution twice as many Äquivalentteilchen (protons here ) Consequently include how molecules of the substance itself
That is, 1 mol / l (H2SO4 ) = 2 N ( H2SO4) or in other words, is a normal 1 ½ molar H2SO4 solution (1 N corresponds to ½ M).
Redox reactions
In redox reactions, however, the equivalent is the amount of substance of the oxidation and reduction agent, which can enter or leave exactly 1 mol of electrons. An example:
Manganese in this reaction, the reducing agent and 1 mol of manganese is 7 mol electrons. Consequently, there is 1/7 mol manganese exactly 1 mol electrons. The Äquivalentteilchen is here 1/7 Mn.
Historical
The use of standard solutions with an equivalent concentration of 1 mol / l ( one normal solution) or 0.1 mol / l was particularly Friedrich Mohr ( 1806-1879 ) introduced in analytical chemistry, especially in his 1855 erschienenem in several editions textbook "Chemical analytical Titrirmethode ".