Phenylmagnesium bromide
Bromo (phenyl ) magnesium
Phenylmagnesium bromide solution purum, ~ 3 M in diethyl ether
Risk
Template: Infobox chemical / molecular formula search available
Phenylmagnesium bromide is a chemical compound from the group of benzene derivatives, and organometallic compounds. As a Grignard reagent, it is usually stable for only a solvent adduct with two equivalents of an ether.
Production and representation
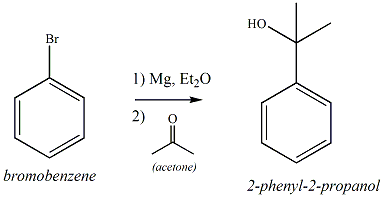
To obtain phenylmagnesium bromide bromobenzene is slowly added to magnesium turnings in dry ether. Once the reaction is started successfully, the ether colored gray - violet and the solution is heated at a faster addition of aromatics to boiling. Due to the ever-present oxide layer on the magnesium, the reaction can be strongly inhibited, so it is often previously etched by adding a few crystals of iodine. Phenylmagnesium bromide, as all Grignard reagents, not isolated in the normal case, but continue to be used in solution.
Due to their high reactivity, it is necessary that when working with phenylmagnesium bromide solutions on a strict exclusion of water - by using a drying tube and before chemical dried ( " ketylierten " ) solvents - is respected, since otherwise form benzene and precipitating in ether magnesium hydroxide. Phenylmagnesium bromide is usually generated in situ and because of its sensitivity to (air) and moisture - not stored carbon dioxide contained in the air a long time - because of the instability to acids. As with all aromatic Grignard reagents as solvent tetrahydrofuran ( THF), diethyl ether which, due to its better stability and thus is preferable Enabled reactivity.
Properties
Is phenylmagnesium bromide, as a so-called Grignard reagent, an important chemical in organic chemistry, at the same time a strong base and is used in Grignard reactions to form new carbon-carbon single bonds.
Usually, it is not isolated as the free substance, but only used as a solution. The stability of solutions of Grignard reagents is based on the coordinate bonding of the lone pairs of the oxygen atom contained in the ether. As butylene "fixed" in THF by the annular structure ( sterically hindered ), and not as the ethyl groups in diethyl ether are freely movable, is explained in the preparation of sterically hindered Grignard reagents, such as (substituted) phenyl or benzyl, the higher stability of the complex in THF, which analogously applies to other intramolecular ether such as 1,4 -dioxane and may be explained by the Schlenk equilibrium. The technical product for laboratory purposes is usually sold as single-, two - or dreimolare solution in tetrahydrofuran or diethyl ether; due to the low boiling point of the diethyl ether, however, the THF - based solutions are preferred, to allow for greater flexibility in the range of the reaction temperature.