Accelerator mass spectrometry
The Accelerator Mass Spectrometry, or AMS ( for closely. Accelerator Mass Spectrometry ) is a form of mass spectrometry. AMS works with a particle accelerator (almost always a tandem accelerator) and two mass spectrometers. In 1977, she was at Oxford University, first developed as a refinement of radiocarbon dating, which is by measuring the radioactive decay of carbon-14 was possible until then. Today is the method for a number of isotopes, usually long-lived radionuclides used. AMS with the ratio of a ( radio ) isotopes is to another - as measured isotope of the same element - usually stable. Worldwide there are about 84 AMS laboratories (July 2006). Most of them are focused on the measurement of 14C.
Taking a Measurement
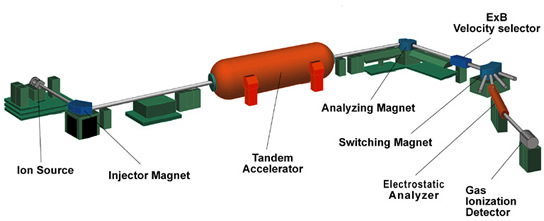
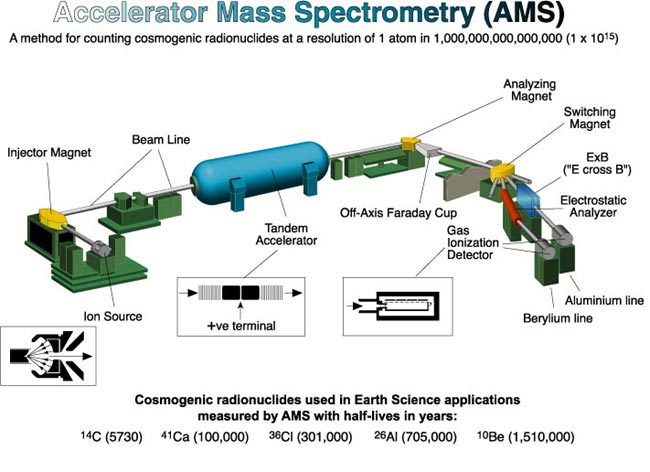
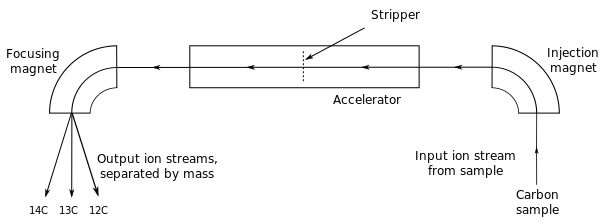
After a chemical treatment of the sample, it is dispersed in the common equipment in a sputter ion source. Here, negatively charged particles formed (anions) are sucked through an electrical high voltage ( several kilovolts ). In a subsequent magnetic field different heavy ions are differentially deflected so that even before the accelerator held a first mass selection. A radiocarbon date would be at this point but not yet possible, because the signal of the measured carbon-14 is still (eg 13C1H or 12C1H2 ) superimposed by a higher many orders of magnitude underground by molecular ions of the same mass. The Isobar stable nitrogen -14 does not form stable negative ions and is therefore not a ground source. This is also the case for the AMS nuclides 26Al and 129I and its stable isobar 26Mg and 129Xe. The same is true for very heavy nuclides such as 240Pu, where no stable isobars exist anymore.
After the first, the above-described composition analysis, the ions are injected in a tandem accelerator. Here they are accelerated by a positive high voltage of several million volts to the so-called terminal to where they fly through a thin carbon foil or a gas channel. There, the ions lose electrons by collisions more electrons. Especially weakly bound outer electrons that are responsible for the binding molecule, are all stripped, so that here, all molecules can be completely destroyed. The now multiple positive charged ions are further accelerated by the same high voltage. After the accelerator, the particle in a magnetic second mass analysis is cleaned of the molecular fragments. The remaining ions in the beam are individually detected by an ionization chamber or a silicon detector, the high particle allowing further drastic background reduction. Thus, measurements of isotopes (eg 10Be, 36Cl, 41Ca, 53Mn, 59Ni, 60FE ) are possible, their stable isobars very well form negative ions, which can then be discriminated against by suitable detector systems but.
The stable isotopes are measured by measuring the current of the ion beam. To the beam in a Faraday cup is collected.
With AMS, for example, 14C/12C-Verhältnisse to about 10-15 measurable. In a typical sample volume of one milligram of this corresponds to a radioactivity of about 0.2 μBq, ie, a disintegrating core in two months. For radiocarbon dating usually the isotope ratio is measured with an accuracy of 0.5%.
Detector systems of the AMS
In the AMS radionuclides are measured by counting in general. Individual ions are registered in particle detectors. There are besides, different detector systems are used.
Silicon detectors
Silicon detectors are semiconductor detectors. They provide an energy signal: the kinetic energy of the incident ion can be measured. A typical application are radionuclides where no isobarischer background is expected. These are, for example, 14C and 26Al, whose stable isobar 14N and 26Mg not form negative ions.
Ionization chambers
Ionization chambers can provide a power signal in the simplest case, or in addition give information about the place or the energy loss.
Ionization chambers, which give information on the energy loss can be the one implemented by radially split anodes ( see also ), on the other hand by the orientation of the electric field in the beam direction and measure the signal rise time. (see Bragg detector )
TOF and ΔTOF
With the abbreviated flight mass spectrometer, also TOFMS ( time-of- flight mass spectrometer ), we determined the ratio mass / charge of the flight time of the ion.
Post- stripping
Post -stripping is a method for the isobars. It is used for example for the measurement of 81Kr. The isobar 81Br is stable. The ion beam is accelerated to a cyclotron to an energy of several GeV and then shot by a film. In the film, the ions pass through many collisions from other electrons. When the power is large enough, there is a portion of the ions from all electrons. Bare nuclei have now a charge corresponding to the order number. Following the post- stripping, the beam is separated with a magnet according to charge. Bromine ions with an atomic number of 35 to achieve the maximum charge 35e, but krypton ions can reach the charge 36e. If the magnet and aperture set so that only ions end up with the charge 36e in the detector, so that the background can be effectively reduced by 81Br.
Gas-filled magnets
Eg for 26Al and 39Ar
Applications
Determine the age of fossils and archaeological finds.