Atomic spectroscopy
Atomic Spectroscopy (also atomic spectrometry ) is a collective term for spectroscopic methods, which are used for quantitative and qualitative determination of chemical elements. Atomic spectroscopy is a part of analytical chemistry. We distinguish:
- Atomic absorption spectrometry ( AAS)
- Atomic emission spectrometry (AES )
- Atomic fluorescence spectroscopy ( AFS)
- Spark spectroscopy ( OES)
- X-ray fluorescence
- Nuclear magnetic resonance spectroscopy (NMR)
Atomic absorption spectrometry ( AAS)
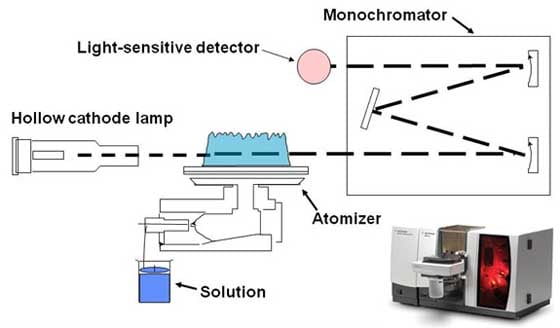
Atomic absorption spectrometry (AAS ) is a proven and fast method for quantitative and qualitative analysis of many elements ( metals, semi- metals) in mostly aqueous solutions and solids. It is based on the attenuation (absorption ) of radiation by interaction with free atoms. Because each chemical element has a characteristic line spectrum can be made to a reference measurement without the sample statements of the elements contained in a sample on the evaluation of the difference spectrum. Atomic absorption spectrometry is divided in terms of introducing a sample into the gas phase in the following sub- processes:
- F- AAS (short for engl. Flame atomic absorption spectrometry, dt, flame atomic absorption spectrometry ', also called flame photometry or flame technique )
- GF- AAS or eM - AAS ( graphite furnace atomic absorption spectrometry german, German, atomic absorption spectrometry with electrothermal heating ', also graphite furnace technique)
- CV- AAS ( atomic absorption spectrometry engl. cold vapor, dt, atomic absorption spectrometry with cold vapor technique ', also called hydride )
- HR-CS AAS (german high-resolution continuum source atomic absorption spectrometry -, dt, atomic absorption spectrometry with continuum source and high-resolution echelle double monochromator ')
Atomic emission spectrometry (AES )
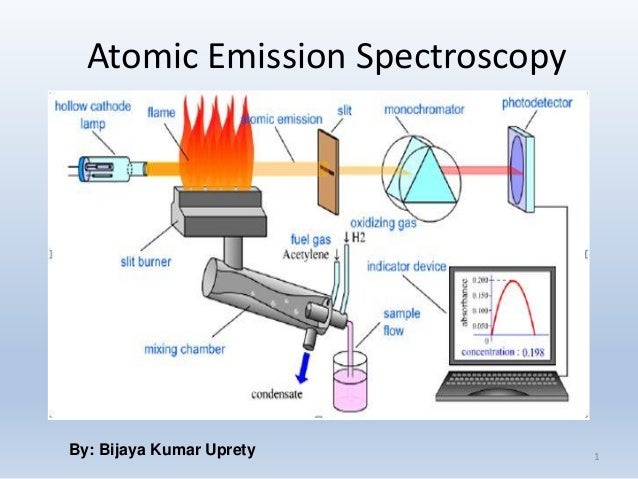
Atomic emission spectrometry (AES ), often also optical emission spectrometry ( OES) as the AAS used for quantitative and qualitative analysis of solid, liquid and gaseous samples.
The method is based on the principle that an excited atom emits a specific element, electromagnetic radiation, and thus provides information about the sample. The excitation of the atoms via an external power supply, such as a flame, arc, spark or an inductively coupled plasma ( ICP), and the transfer into the plasma state. Therefore, the following sub- processes yield:
- Flame atomic emission spectrometry ( engl.: flame atomic emission spectrophotometry, F -AES)
- Optical emission spectrometry with inductively coupled plasma (English inductively coupled plasma optical emission spectrometry, ICP -OES)
- Microwave plasma torch - AES (English microwave plasma torch atomic emission spectrometry MPT -AES)
Atomic fluorescence spectroscopy ( AFS)
Both fluorescence and phosphorescence are types of luminescence ( cold light fixtures). Fluorescence, however, is characterized in that it quickly (usually within a millionth of a second ) ends after the end of irradiation. In phosphorescence, however, there is an afterglow that can last from a fraction of a second to hours.
Atomic Fluorescence is the emission of optical accommodated in the gas phase atoms and which have been brought about by the absorption of electromagnetic radiation (e.g., photons) to a higher energy state. The main advantage over fluorescence spectroscopy AAS, the greater sensitivity that is permitted by the lower background noise. The resonant excitation method provides a selective excitation of the analyte to avoid interference. The AFS allows the examination of the electronic structure of atoms and quantitative measurements. An analysis of solutions or solid materials requires that the analyte is dissolved, vaporized and atomized. This is accomplished in a heat pipe or a flash or graphite furnace it should be done at relatively low temperatures. The actual stimulation of the atoms is carried out through a hollow cathode lamp ( HCV ), or by a laser. The detection is similar to that of atomic emission spectroscopy using monochromators and photomultiplier.
Inductively coupled plasma mass spectrometry (ICP -MS)
ICP- MS stands for inductively coupled plasma mass spectrometry, ie mass spectrometry, inductively coupled plasma. In contrast to the previous techniques is observed in ICP -MS is not absorbed or emitted by atoms of light, but the impact of ions and their mass is measured on a detector.
A liquid sample is sucked by a pump, atomized in an atomizer and bruised in an argon plasma in the so-called flare or torch and ionized. The mostly singly charged ions are focused in high vacuum by means of an electric lens optics, separated in a quadrupole according to their mass / charge ratio and then impinge on a detector which records the number of ions per unit mass and thus allows a quantitative analysis of the elements.
The ICP -MS combines the capability of a multi-element analysis and the wide linear working range of the ICP -OES with the very good detection limits of the graphite furnace AAS and exceeds them. It is also one of the few analysis techniques, which allows the quantification of isotopic concentrations in the analysis of the elements. However, may be introduced because of the bottleneck at the transition from argon plasma for high vacuum limited salt loads. Expensive high-resolution devices (sector field devices with resolutions up to 10,000 ) may differ Atom-/Molekülmassen in the decimal range; thus they separate molecular interferences from the actually measured element masses. Your sensitivity is a further increased over the Quadrupolgeräten until 1000. They are suitable because of their low material consumption therefore also well suited for analysis of radioactive samples.
Nuclear resonance spectroscopy
The method is based on the nuclear magnetic resonance of a resonant interaction between the magnetic moment of atomic nuclei of the sample is in a strong static magnetic field with a high frequency alternating magnetic field. It is only those isotopes spectroscopy available which have a non-zero nuclear spin and therefore a magnetic moment in the ground state.