Bead test
The borax bead is used as a preliminary test on some transition metals in the separation process in analytical chemistry.
For this purpose, a previously annealed magnesia borax ( Na2B4O7 10 H2O) is added and melted in the Bunsen burner flame to a crystal clear pearl. Here, the crystal water contained escapes. A small amount of the powdered sample is then received and retained in the burner flame until the bead is completely melted again and the sample was evenly distributed therein with this pearl. Then observed the color of the pearl in both the heat and the cooled state.
Depending on the flame zone used ( light = reducing flame, non-luminous flame, oxidation ), there are different colors of the pearl. These are largely identical to the staining in the microcosmic salt bead.
Reactions in the pearl
Chemically arise seen in the heat of heavy metal borates. These have different composition.
Step 1: annealing of the hydrous borax:
Step 2: Formation of the heavy metal borate exemplified by cobalt (II) -sulfate:
Risk assessment
Borax (sodium tetraborate ) has now been classified as toxic to reproduction and potentially teratogenic. Since there is the salt of phosphorus bead a less dangerous and equivalent, from an analytical point of view alternative, is should not be used for the purposes of the appropriate laboratory test to dealing with borax.
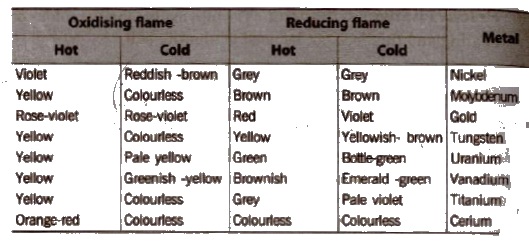
Colors in the borax bead
The color is contained by the cation -dependent and can be a clear indication of the corresponding metal. In mixed samples but can often be closed only on the presence or absence of certain strong coloring ions (eg, Co2 , Cr3 ), since it can also lead to mixed colorings or for covering less intense staining.
As proof, the borax bead is therefore not sufficient. The borax bead obtained by the notice must be confirmed by appropriate detection reactions.