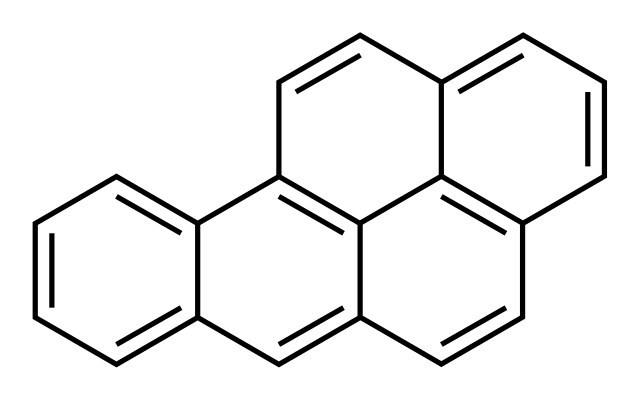
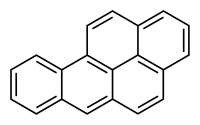
Benzo(a)pyrene
Yellowish, aromatic scent from her substance
Fixed
1.28 g · cm -3 ( 20 ° C)
180 ° C.
495.5 ° C
Practically insoluble in water, soluble in organic solvents
Risk
Not classified as carcinogenic effect
Template: Infobox chemical / molecular formula search available
Benzo [ a] pyrene ( 1,2- benzopyrene ) is a polycyclic aromatic hydrocarbon. The obsolete term for this chemical compound is 3,4- benzopyrene ( IUPAC name: benzo [ pqr ] tetraphen ).
Occurrence and formation
Benzo [a ] pyrene is found in coal tar. In addition, it results from the incomplete combustion of organic substances and is consequently widely used. So you can find it in auto and industrial emissions. During smoking of cigarettes is formed at about 300 ° C in the tobacco burning zone, but also in the roasting of coffee beans, small quantities ( 0.3 to 0.5 ug · kg -1).
In Grill products that have been cooked over charcoal or pine cones, can be benzo [a ] pyrene detected. Traces of benzo [a ] pyrene are found in soil, vegetables and grains.
Production and representation
Benzo [a ] pyrene can be obtained from coal tar and other tars by extraction. An early synthesis variant described in 1935 starts with a Friedel -Crafts acylation of pyrene with succinic anhydride. The resulting Pyrenoylpropionsäure is reduced by zinc to Pyrenylbuttersäure and then cyclized in the presence of tin tetrachloride. The intermediate is then reduced again by Ketotetrahydrobenzopyren zinc to Tetrahydrobenzopyren. Pyrene the formation of the benzo [a] is then carried out by dehydration by means of sulfur or selenium. A recent three-step synthesis is based on the 2- naphthyl boronic acid, which is coupled ,3- dicarbaldehyde in a Suzuki reaction with 2- bromobenzene -1 in the first step. The aldehyde of the coupling product can be reacted in the second step in a Wittig reaction with Methoxymethylentriphenylphosphin. The cyclization to the target compound is then performed using methane sulfonic acid.
Structure
Benzo [a] pyrene molecule belongs to the penta -nuclear, that is, consisting of five benzene rings, polycyclic aromatic hydrocarbons. The rings are connected to one another via their edges ( fused ). The molecule possesses, such as benzene, due to the delocalized π - electron system, aromatic character.
Properties
Benzo [ a] pyrene is a yellow, crystalline solid, which is in the form of flakes having a density of 1.282 g · cm -3 or needles ( density 1.351 g · cm -3) either. Benzo [ a] pyrene, melts at 179 ° C and boils at 495 ° C. The vapor pressure function is obtained after August according to log ( P) = A / T B (P in Pa, T in K) where A = 4989 and B = 11.59 in a temperature range of 70 ° C to 180 ° C. In water and alcohols, it is un - or low solubility. In aromatic hydrocarbons, however well it dissolves in benzene, for example, under violet fluorescence.
Safety
Benzo [ a] pyrene is a TRK - value of 0.002 mg / m³. The KOC value is 4,500,000 l / kg (sediment) and the log KOW value of 6.15.
Carcinogen
Benzo [a ] pyrene is one of the longest known and studied cancer-causing ( carcinogenic ) substances. The risk that cigarette smoke causes lung cancer, to a large extent on the benzo [ a] pyrene recycled. Benzo [a ] pyrene is also regarded as the cause of the so-called chimney sweeps cancer, a tumor of the testicle skin, which is developed mainly by the irritation of the soot in the pyrene Benzo [ a] is included. Benzo [a ] pyrene itself is not present toxic, but is in the body by monooxygenases of the cytochrome P450 family ( CYP1A1 and CYP1B1 subtypes ) first converted pyrene -7 ,8- epoxide to benzo [a]. After hydrolysis of the epoxy group by an epoxide hydrolase, the resulting 7,8- dihydroxybenzo [a] pyrene is epoxidized in the 9 position and then again to the carcinogenic benzo [a] pyrene -7 ,8 -dihydroxy- 9 ,10- epoxy implemented. The epoxy group reacts chemically with DNA (specifically guanine ) and thus damaging the structure of DNA, which can prevent cell division or favor mutations.
Ecology
It is a prototype of polycyclic aromatic hydrocarbons. In determining the environmental impact of this group of substances is usually benzo [ a] pyrene can be used as a reference. Benzo [a ] pyrene is named after its danger potential to be toxic and hazardous to the environment.
Proof
Benzo [ a] pyrene can be detected using fluorescence spectroscopy. From mixtures with other compounds, it can be isolated by thin layer chromatography or HPLC.

pyrene-3D-balls-2.png)
pyrene_metabolism.svg/540px-Benzo(a)pyrene_metabolism.svg.png)




pyrene.png)