Bredt's rule
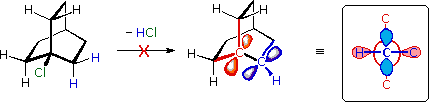
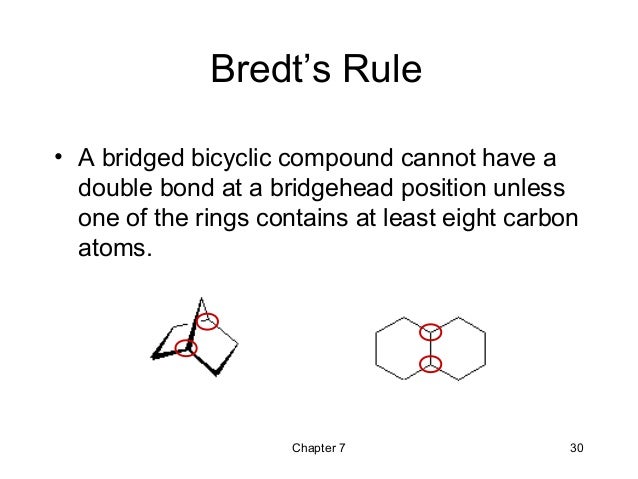
The Bredt rule is an empirical rule in organic chemistry. First they put the German chemist Julius Bredt in 1924. It says that in small bicyclic hydrocarbons due to the ring strain no double bond may be due to a bridgehead atom.
Meanwhile, the Bredt rule has been clarified. Accordingly, only those bicyclo [ xyz] alk -1-enes are stable, in which the sum S of the bridge members meets the following requirement: S = x y z ≥ 7
However, some unstable bicyclic hydrocarbons with S = 5 or 6 could be detected indirectly by secondary reactions.
Figuratively, one can imagine that a double bond at the bridgehead would correspond to a trans double bond, for ring systems with less than eight members is unlikely to occur due to the resulting ring strain. As a result of the geometry of the overlap of those involved in binding the p orbitals of the sp2 -hybridized carbon atoms would be too low to ensure a stable bond. Similar to trans double bonds in cyclic hydrocarbons occur double bonds at the bridgehead of bicyclic compounds on until a size of eight ring atoms.
In addition, there are some compounds that do not follow the Bredt rule. These are also referred to as anti -Bredt compounds. One example is synthesized by Philip E. Eaton and Charles L. Hoffmann 1 (9 ) - homocubene, where the term of the olefin herein pursuant to theoretical studies can be applied only to a limited - due to the strong distortion of the p- orbitals, which the π - realize binding. The 1 (9 ) - homocubene is currently considered the olefin with the strongest twist.